Mass Spectrometry of Fatty Acid Pyrrolidides
Monoenoic Fatty Acids
Straight-Chain C18 Monoenoic Fatty Acids
 Details
of the mass spectra of pyrrolidides of a comprehensive series of monoenoic fatty acids have been published
(Andersson and Holman, 1974), including all the cis-octadecenoic isomers from 4- to 17-18:1,
but very few of the mass spectra were illustrated, although data for the key ions were tabulated.
Those used in this study were synthesised by Gunstone and Ismail (1967).
When faced with the spectrum of an unknown fatty acid, I much prefer to have that of an authentic sample in front of me for comparison purposes,
and therefore I illustrate the spectra of all the available isomers here.
It is worth noting that in interpreting the mass spectra of pyrrolidides of polyenoic fatty acids,
it is the position of the first double bond that is always hardest to locate from first principles,
especially if it is relatively close to the carboxyl group,
and a comparison with spectra of standard monoenes (treated as ‘fingerprints’) can be useful.
If all else fails, pyrrolidides and DMOX derivatives often have very similar spectra, though not when double bonds are adjacent to either
end of the molecule, when once more it may be essential to make comparisons with authentic spectra.
It is not possible to determine the geometry of double bonds with any of these derivatives.
Details
of the mass spectra of pyrrolidides of a comprehensive series of monoenoic fatty acids have been published
(Andersson and Holman, 1974), including all the cis-octadecenoic isomers from 4- to 17-18:1,
but very few of the mass spectra were illustrated, although data for the key ions were tabulated.
Those used in this study were synthesised by Gunstone and Ismail (1967).
When faced with the spectrum of an unknown fatty acid, I much prefer to have that of an authentic sample in front of me for comparison purposes,
and therefore I illustrate the spectra of all the available isomers here.
It is worth noting that in interpreting the mass spectra of pyrrolidides of polyenoic fatty acids,
it is the position of the first double bond that is always hardest to locate from first principles,
especially if it is relatively close to the carboxyl group,
and a comparison with spectra of standard monoenes (treated as ‘fingerprints’) can be useful.
If all else fails, pyrrolidides and DMOX derivatives often have very similar spectra, though not when double bonds are adjacent to either
end of the molecule, when once more it may be essential to make comparisons with authentic spectra.
It is not possible to determine the geometry of double bonds with any of these derivatives.
Our web page on mass spectrometry pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.).
In discussing the interpretation of the mass spectra of pyrrolidide derivatives of monoenoic fatty acids, the task is simplified by first considering the spectrum of the most abundant of all monoenes, oleate (9-18:1). Therefore, the electron-impact mass spectrum of N‑octadec-9-enoyl-pyrrolidine is -
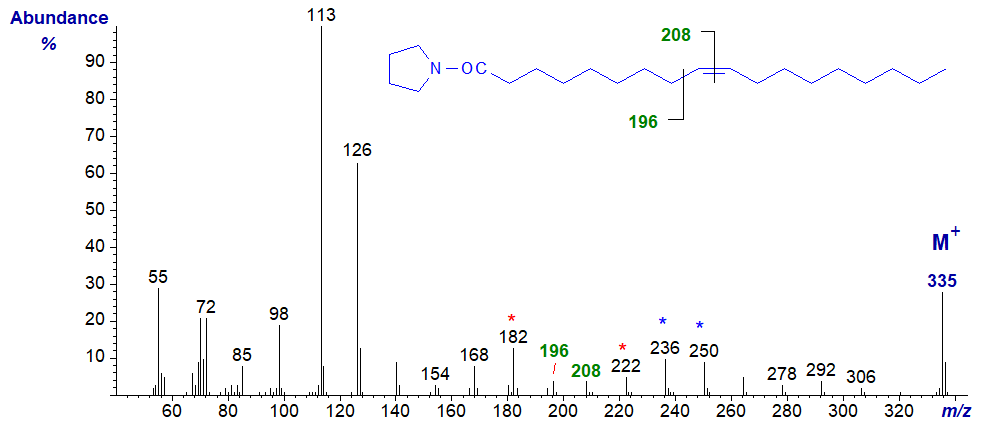
Magnification of the ions in the high mass range relative to the base ion is helpful in making the key diagnostic ions more visible, but unlike the situation with saturated derivatives it is not essential. As well as the base ion at m/z = 113, that at m/z = 126 (both observed with saturated and most other fatty acid pyrrolidides) and the molecular ion, there is a series of ions 14 amu apart in general, except in the vicinity of the double bond, where there is an interval of 12 amu, i.e., occurring between m/z = 196 and m/z = 208, and corresponding to fragmentation between carbons 8 and 9 in the aliphatic chain. For the spectra of the pyrrolidides of the isomeric 6- to 15‑octadecenoic acids (18:1 isomers and not the full range), the following rule was formulated:
"If an interval of 12 amu, instead of the regular 14, is observed between the most intense peaks of clusters of fragments containing n-1 and n carbon atoms in the acyl moiety, a double bond occurred between carbons n and n+1 in the molecule".
This is exactly the same as was described later for DMOX derivatives and is discussed in the appropriate page of this website (DMOX derivatives of monoenoic fatty acids). Of course, the rule can be applied to a wider range of isomers other than C18 in chain length, but when a double bond is near an end of the molecule, it is advisable to compare with spectra of model compounds. With real samples, a noisy baseline or an overlapping peak can confound identification from first principles, so it is always helpful to view authentic spectra. It is my general impression that the diagnostic gap of 12 amu may not be quite as distinctive with pyrrolidides as with DMOX derivatives.
Two other useful ions in the spectrum of the oleate derivative, though less well documented in the literature, are those at m/z = 236 and 250, which stand out a little above those at either side and are formed by rearrangement and cleavage in the beta and gamma positions relative to the double bond. They tend to be seen most clearly when the double bonds are in the distal half of the molecule and in longer-chain derivatives. In addition, the ions on either side of the diagnostic ions, in this instance at m/z = 182 and 222 and 40 amu apart, are often easier to find unambiguously, and this can be especially important when examining spectra of dienes and polyenes.
The mass spectrum of the pyrrolidide of 2-octadecenoate or 2-18:1 is illustrated next (in this instance, the trans isomer, although it would not be expected to differ from the cis isomer). This has not been published formally elsewhere.
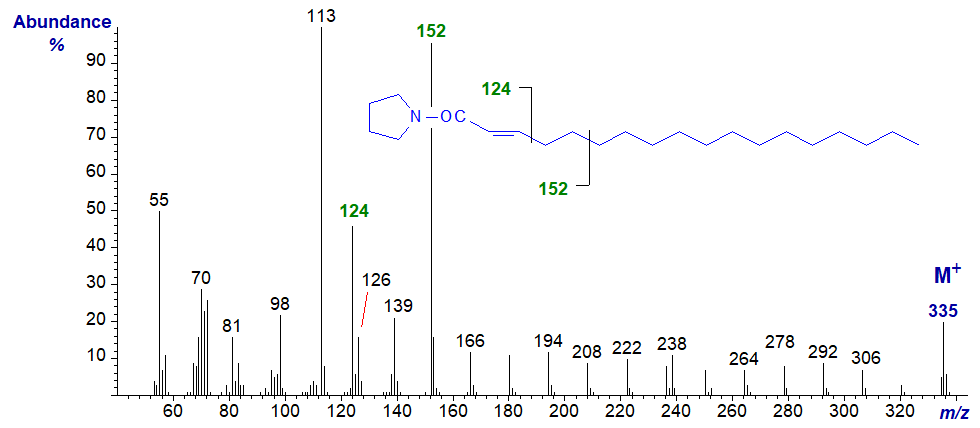
The expected ion at m/z = 126 is of low abundance, and instead an ion at m/z = 124 stands out, presumably representing cleavage between carbons 3 and 4 as indicated in the figure. The distinctive and defining feature is an especially abundant ion at m/z = 152, which can be considered simplistically as representing cleavage as shown (possibly after formation of a stable conjugated double bond system). Thereafter, the remaining ions are 14 amu apart as expected. In this instance, the spectrum is very different from that of the corresponding DMOX derivative. Although this fatty acid may not occur in nature, it can be formed inadvertently from fatty acids with a double bond in position 3 during the derivatization reaction (see below).
Monoenoic fatty acids with double bonds in position 3 (trans-configuration) are found in the photosynthetic tissues of plants. I was unable to prepare the pyrrolidide of synthetic cis-3-octadecenoate by the usual reaction of the methyl ester derivative with pyrrolidine and acetic acid (see our web pages on Preparation of derivatives for mass spectrometry). Instead, it appeared to be converted entirely to the 2‑isomer, presumably because the strong base caused isomerization. This is the only adverse reaction that we have found so far for pyrrolidides. In this instance, the derivative was prepared successfully by reaction of the acid chloride with pyrrolidine. On the other hand, it was a surprise to find that the trans-3 isomer (in a polyunsaturated fatty acid from a seed oil) was derivatized with only minimum isomerization to the 2-isomer, from which it is well separated by gas chromatography.
The mass spectrum of the pyrrolidide of trans-3-octadecenoate (3t-18:1) -
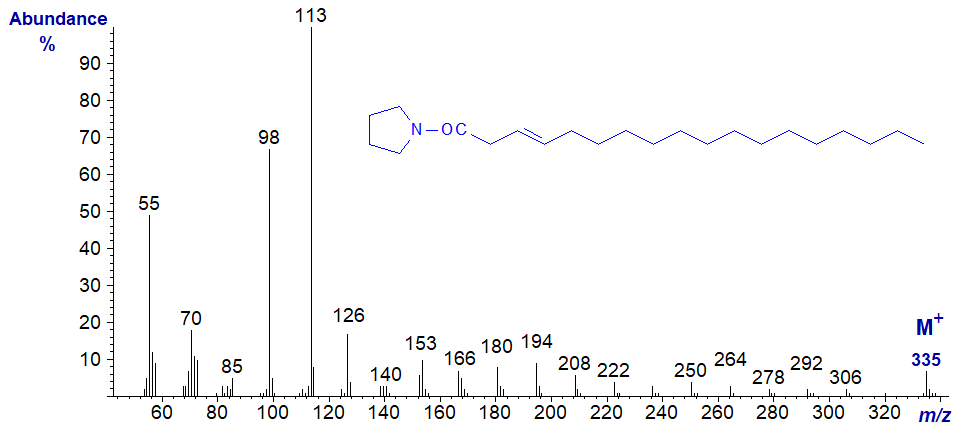
There appear to be no ions that stand out as markers to distinguish it from other isomers, although paradoxically this feature alone makes the spectrum distinctive as a ‘fingerprint’ at least. From m/z = 166 upwards, the significant ions are all 14 amu apart confirming that the double bond is indeed close to the carboxyl group. Access to the standard spectrum may assist readers faced with an unknown, as once more this differs appreciably from the spectrum of the DMOX derivative.
The mass spectrum of the pyrrolidide of 4-octadecenoate (4-18:1) -
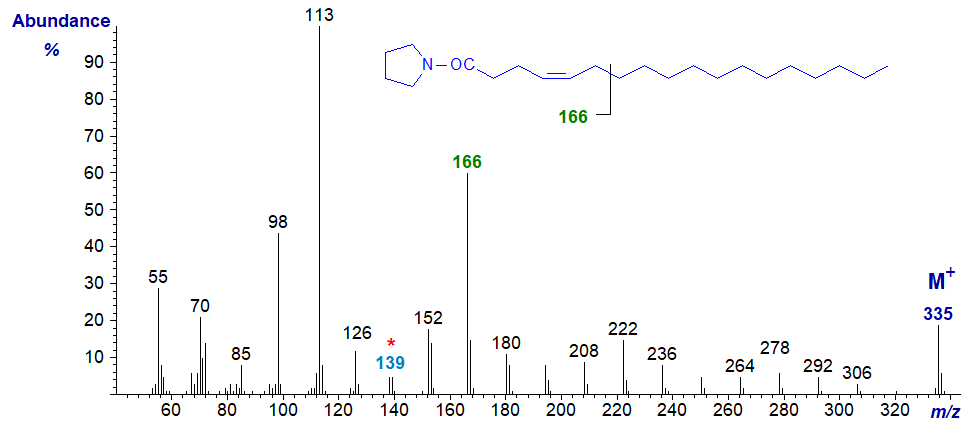
While it is possible to seek out ions for the gap of 12 amu 'typical' of monoenes, I find in practice that the simplest approach is to consider the spectrum again as a fingerprint. The ions at m/z = 138/139 and 166 are distinctive and following the ion at m/z = 152, the main ions in each cluster are 14 amu apart confirming that the double bond must be close to the carboxyl group.
The mass spectrum of the pyrrolidide of 5-octadecenoate (5-18:1) -
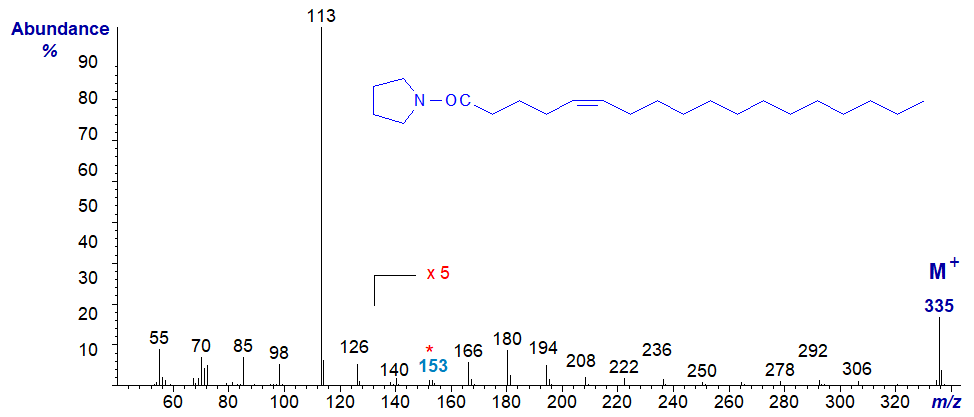
In this instance, the ions in the higher mass range are all of very low intensity relative to the base ion, even when magnified five-fold as here. This feature and a very small ion at m/z = 153 must be considered part of the distinctive fingerprint for all acyl-pyrrolidides with a double bond in position 5 (and it is also seen with DMOX derivatives).
The mass spectrum of the pyrrolidide of 6-octadecenoate (6-18:1 or petroselinate) -
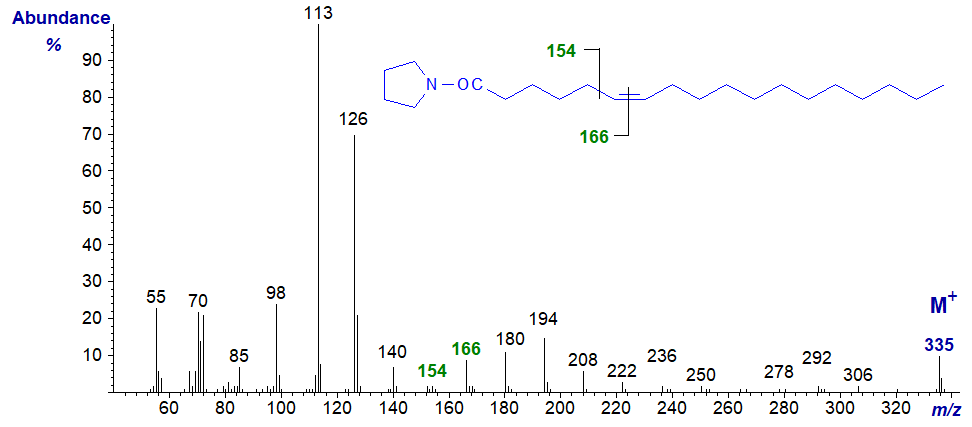
This spectrum resembles that of the 5-isomer, although the distinctive ions in the higher mass region can be seen without magnification. The key to recognition is the small ion at m/z = 154 (compared to 153 for the 5-isomer) and the gap of 12 amu to the ion at m/z = 166 that serves to locate the double bond. With an unknown component in a natural sample, I would prefer to have additional information from an alternative derivative to be certain.
The mass spectrum of the pyrrolidide of 7-octadecenoate (7-18:1) -
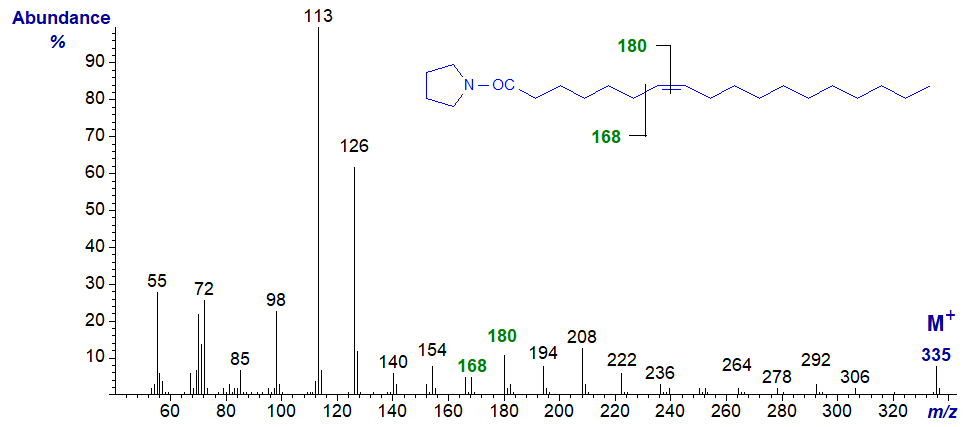
The 'rule of the gap of 12 amu' now really starts to apply, and the ions at m/z = 168 and 180 locate the double bond.
The mass spectrum of the pyrrolidide of 8-octadecenoate (8-18:1) -
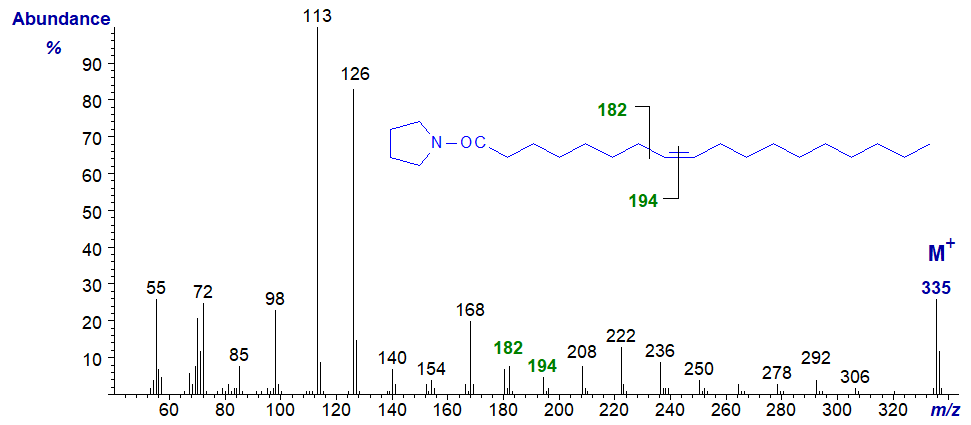
- now, the gap of 12 amu for the double bond is between m/z = 182 and 194.
The mass spectrum of the pyrrolidide of 10-octadecenoate (10-18:1) -
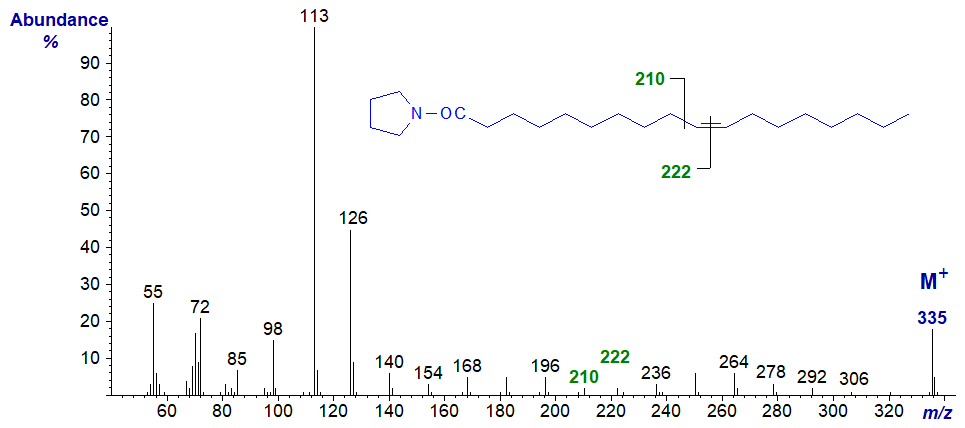
- the gap of 12 amu for the double bond is between m/z = 210 and 222.
The mass spectrum of the pyrrolidide of 11-octadecenoate (11-18:1 or cis-vaccenate) -
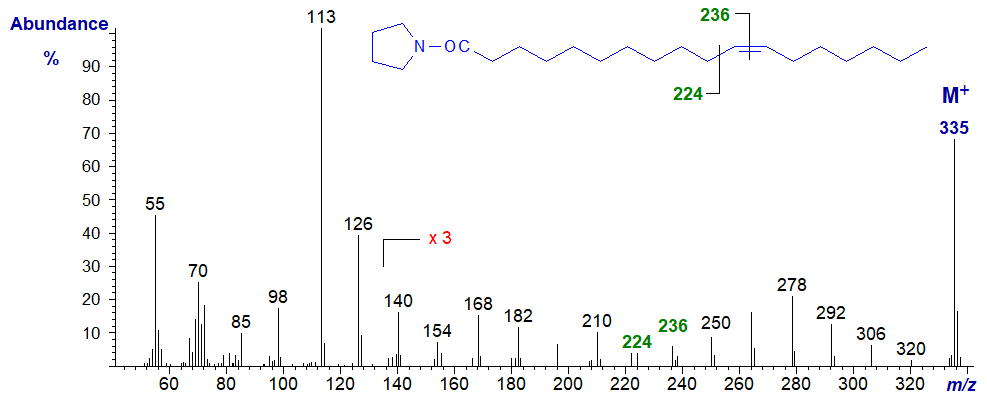
- the gap of 12 amu for the double bond is between m/z = 224 and 236.
The mass spectrum of the pyrrolidide of 12-octadecenoate (12-18:1) -
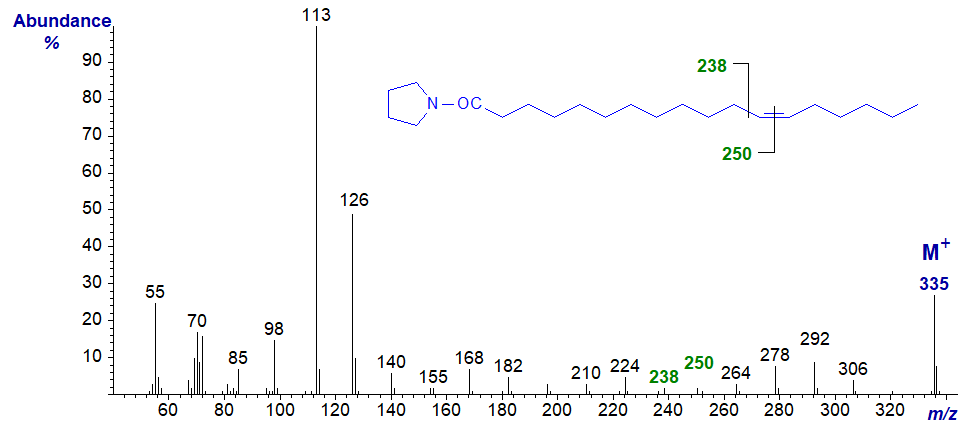
- the gap of 12 amu for the double bond is between m/z = 238 and 250.
The mass spectrum of the pyrrolidide of 13-octadecenoate (13-18:1) -
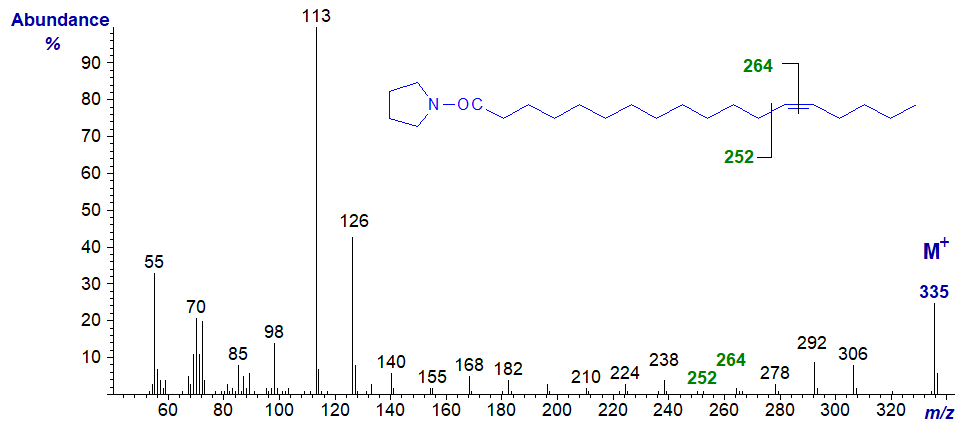
- the gap of 12 amu for the double bond is between m/z = 252 and 264.
The mass spectrum of the pyrrolidide of 14-octadecenoate (14-18:1) -
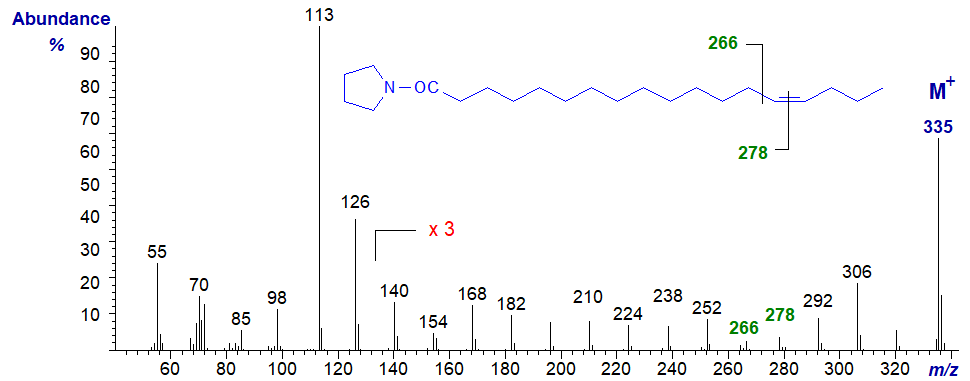
- the gap of 12 amu for the double bond is between m/z = 266 and 278.
The mass spectrum of the pyrrolidide of 15-octadecenoate (15-18:1) -
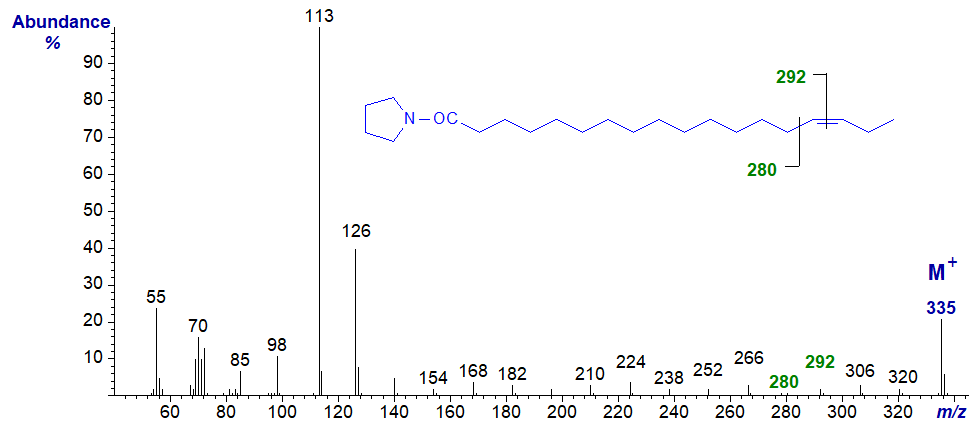
- the gap of 12 amu for the double bond is between m/z = 280 and 292.
The mass spectrum of the pyrrolidide of 16-octadecenoate (16-18:1) -
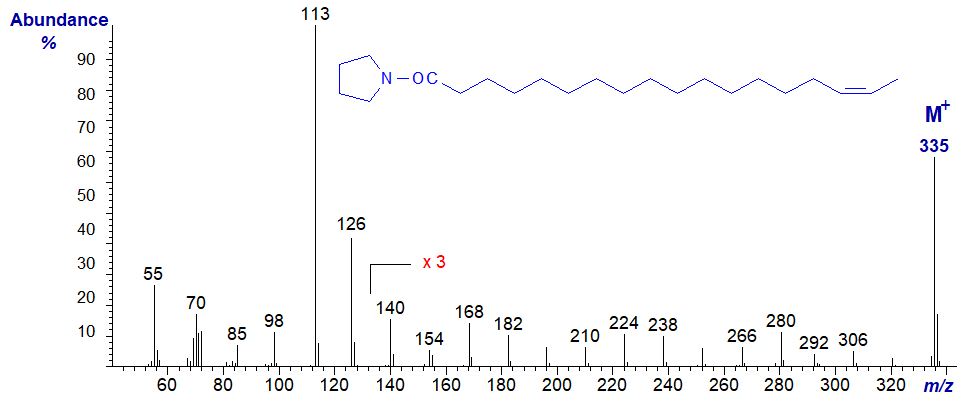
The spectrum should be considered as that of an isomer with a double bond in the penultimate position, rather than being typical of a double bond in position 16 in all circumstances, and the formal rule described above for locating double bonds no longer applies. In this instance, the spectrum is almost identical to that of the 15‑18:1 isomer. The only significant difference is that the ion at m/z = 280 is more abundant than that at m/z = 266, while the reverse is true for the 15-isomer.
The mass spectrum of the pyrrolidide of 17-octadecenoate (17-18:1) -
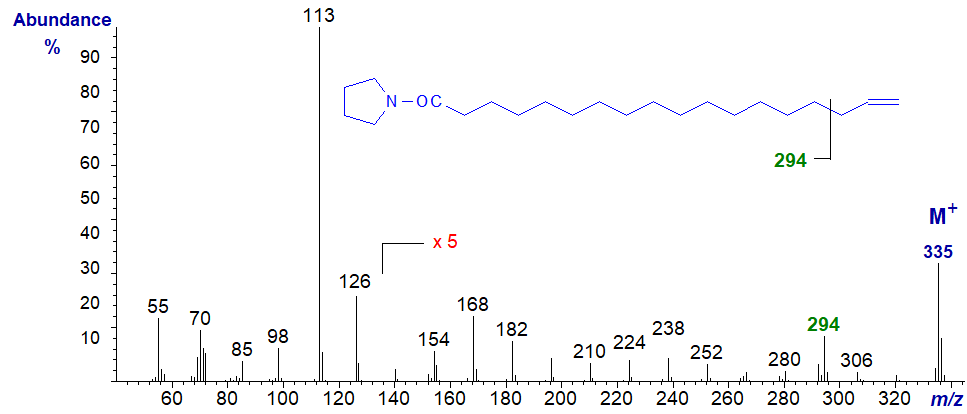
Here, it was necessary to magnify the spectrum five-fold to see the key diagnostic ions in the high mass region. For an isomer with a terminal double bond, the ion at m/z = 294, which is presumably formed by cleavage beta to the double bond (between carbons 15 and 16) is a useful diagnostic guide. Of course, this should be considered as the spectrum of a fatty acid with a terminal double bond rather than simply as one with the double bond in position 17. A longer-chain fatty acid with a double bond in position 17 would be expected to follow the general rule of 'the gap of 12 amu'.
Straight-chain Monoenoic Fatty Acids of Other Chain-Lengths
The rules described above apply for the determination of double bonds in fatty acids of other chain lengths. For comparison with the last, the mass spectrum of the pyrrolidide of 17-hexacosenoate (17-26:1) from a seed oil -
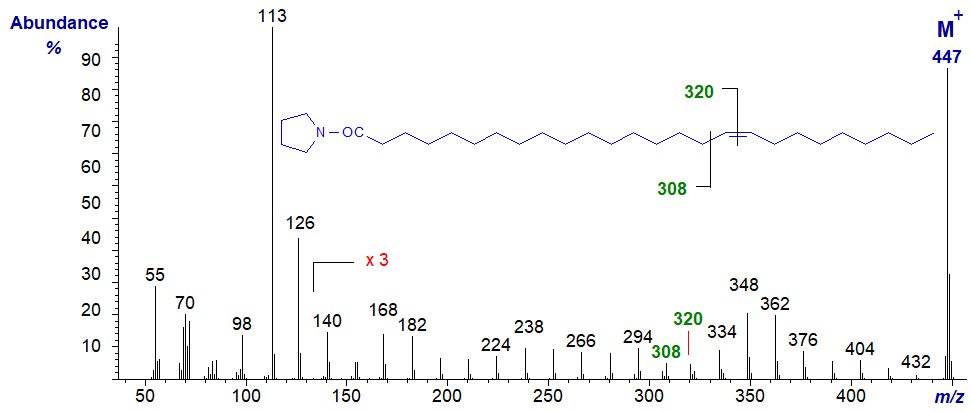
- here, the double bond is located by the gap of 12 amu between m/z = 308 and 320, as expected, i.e., the double bond is now centrally located rather than in the terminal position and the characteristic fragments are those anticipated for a central double bond.
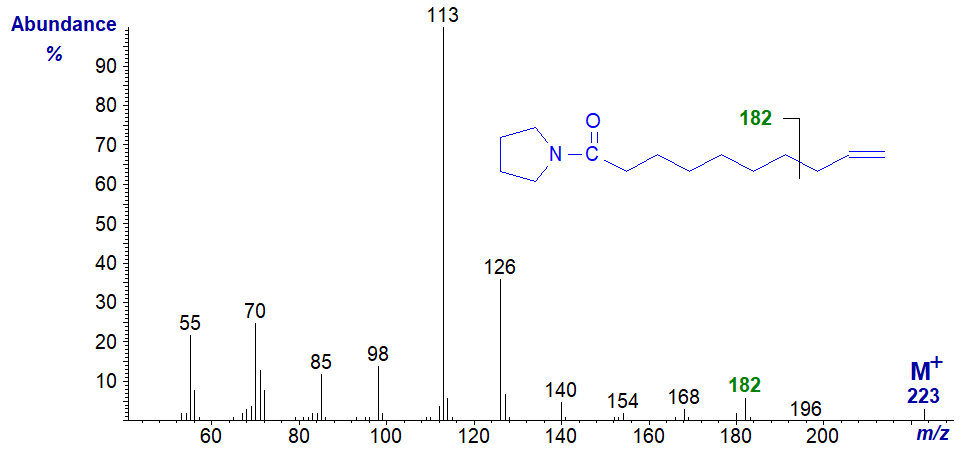
On the other hand, with the mass spectrum of the pyrrolidide of 9-decenoate (9-10:1) from milk fat, in which the double bond is terminal, we have the anticipated fragmentation for a terminal double bond, i.e., the fragment ion beta to the double bond at m/z = 182 and not the ions expected for a double bond in position 9.
Mass spectra of pyrrolidides of many more monoenoic acids of differing chain lengths can be found in our Archive Section, but without interpretation. Most of these have not been published elsewhere.
Branched-Chain Monoenoic Fatty Acids
The mass spectrum of the pyrrolidide of 7-methyl-hexadec-6-enoate, often encountered as a minor component of fish oils, in this case from Aurelia sp. (a jelly fish - sample courtesy of Peter Nichols in Tasmania) -
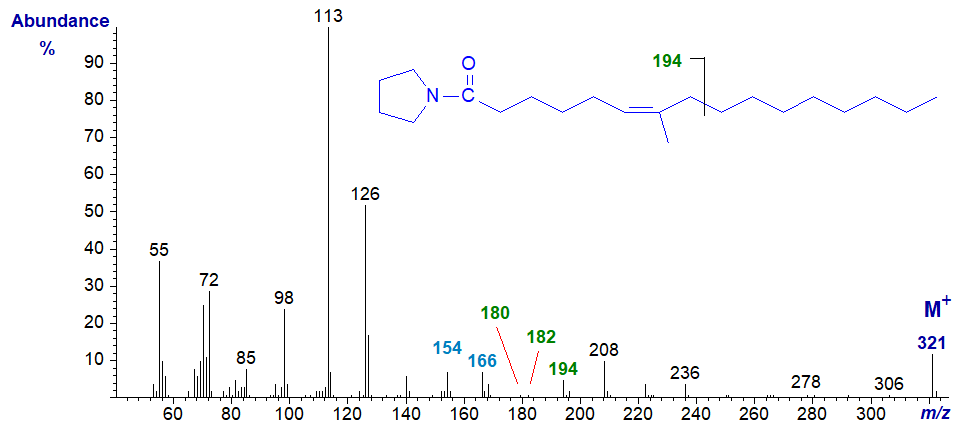
Both the cis and trans isomers of this fatty acid are known, and they have indistinguishable mass spectra. Other positional isomers occur, but the key diagnostic feature in this instance is apparently the small ion at m/z = 180, together with the 12 amu difference between the fragments at m/z = 154 (C5) and m/z = 166 (C6), which locate the double bond in position 6 (Carballeira and Shalabi, 1994).
References
- Andersson, B.A. and Holman, R.T. Pyrrolidides for the mass spectrometric determination of the position of the double bond in monounsaturated fatty acids. Lipids, 9, 185-190 (1974); DOI.
- Carballeira, N.M. and Shalabi, F. Unusual lipids in the Caribbean sponges Amphimedon viridis and Desmapsamma anchorata. J. Nat. Prod., 57, 1152-1159 (1994); DOI.
- Gunstone, F.D. and Ismail, I.A. Fatty acids. Part 13. The synthesis of all the cis n-octadecenoic acids. Chem. Phys. Lipids, 1, 209-224 (1967); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
