Mass Spectrometry of 3-Pyridylcarbinol Esters
Cyclic Fatty Acids
 This
document does not aim to be a complete account of mass spectrometry with electron-impact
ionization of naturally occurring fatty acids containing ring structures as the 3-pyridylcarbinol ('picolinyl') esters,
but rather is a personal account of our experience of those encountered during our research activities
and for which we have spectra available for illustration purposes.
Spectra of these fatty acids for methyl esters
and DMOX/pyrrolidide derivatives are described in separate documents.
Where we are aware of prior illustrations of mass spectra in the literature, the appropriate papers are cited.
These notes are intended as a practical guide rather than as a mechanistic account,
but our web page on mass spectrometry of 3‑pyridylcarbinol esters of saturated fatty acids
contains more introductory and mechanistic information,
together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.)
The occurrence and biological properties of cyclic fatty acids have been reviewed by Sébédio, J.L. and Grandgirard, A.
(1989), and there is further information on the chemistry, occurrence and biochemistry of natural cyclic fatty acids on this website
here...
This
document does not aim to be a complete account of mass spectrometry with electron-impact
ionization of naturally occurring fatty acids containing ring structures as the 3-pyridylcarbinol ('picolinyl') esters,
but rather is a personal account of our experience of those encountered during our research activities
and for which we have spectra available for illustration purposes.
Spectra of these fatty acids for methyl esters
and DMOX/pyrrolidide derivatives are described in separate documents.
Where we are aware of prior illustrations of mass spectra in the literature, the appropriate papers are cited.
These notes are intended as a practical guide rather than as a mechanistic account,
but our web page on mass spectrometry of 3‑pyridylcarbinol esters of saturated fatty acids
contains more introductory and mechanistic information,
together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.)
The occurrence and biological properties of cyclic fatty acids have been reviewed by Sébédio, J.L. and Grandgirard, A.
(1989), and there is further information on the chemistry, occurrence and biochemistry of natural cyclic fatty acids on this website
here...
Cyclopropyl Fatty Acids
Cyclopropyl fatty acids are common constituents of bacterial lipids and may accompany cyclopropene fatty acids as minor components of certain seed oils. 3‑Pyridylcarbinol esters are by far the most useful derivatives for characterization of fatty acids with cyclopropane rings, as they give distinctive cleavages that permit facile location of the ring in the alkyl chain (illustrated simplistically here). The mass spectrum of 3‑pyridylcarbinyl 9,10-methylene-octadecanoate is -
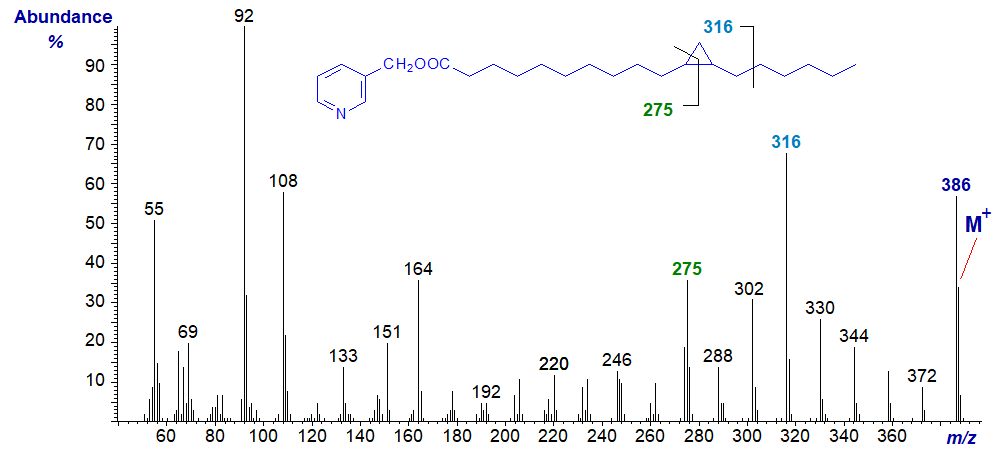
There are the usual ions in the lower molecular weight region at m/z = 92, 108, 151 and 164, typical of a 3-pyridylcarbinol ester, but the [M‑1]+ ion is more abundant than the molecular ion (m/z = 387) itself. The distinctive ion that permits location of the ring is odd-numbered (uncommon) at m/z = 247, representing cleavage at the ring as shown (Harvey, D.J., 1984). Otherwise, the homologous series of ions 14 amu apart from m/z = 372 down to 274 confirm that there are no unusual features between C10 and C17, while a comparable homologous series of ions in the early part of the spectrum is of similar value.
With methyl esters and dimethyloxazoline and pyrrolidide derivatives of cyclopropyl fatty acids, the ring appears to rearrange under electron bombardment in the mass spectrometer so that spectra are almost identical to those of monoenoic fatty acids with an alkyl chain one carbon longer. While these can still be useful, 3‑pyridylcarbinol derivatives are to be preferred. Alternative procedures use ring-opening methods prior to mass spectrometry (see our web page on methyl esters of cyclic fatty acids).
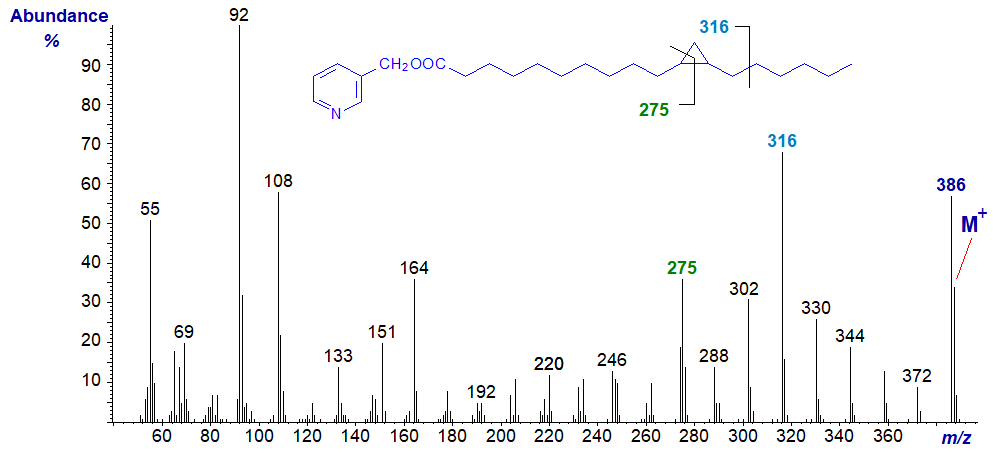
In the spectrum of 3-pyridylcarbinyl 11,12-methylene-octadecanoate (lactobacillic acid), illustrated next, the distinctive ion that locates the ring has shifted 28 amu as expected to m/z = 275.
Cyclopropenyl Fatty Acids
It was long thought that gas chromatography (GC) and thus GC-MS of derivatives of cyclopropenyl fatty acids was impossible because of thermal degradation or isomerization of the sensitive functional group on the GC column, but modern capillary columns are relatively inert, and analysis by GC is straightforward provided that appropriate derivatization methods are employed, i.e., that acidic conditions are avoided.
The mass spectrum of 3-pyridylcarbinyl sterculate (from the seed oil of Sterculia africana) is distinctive and is illustrated next (see Spitzer, V. et al., 1994).
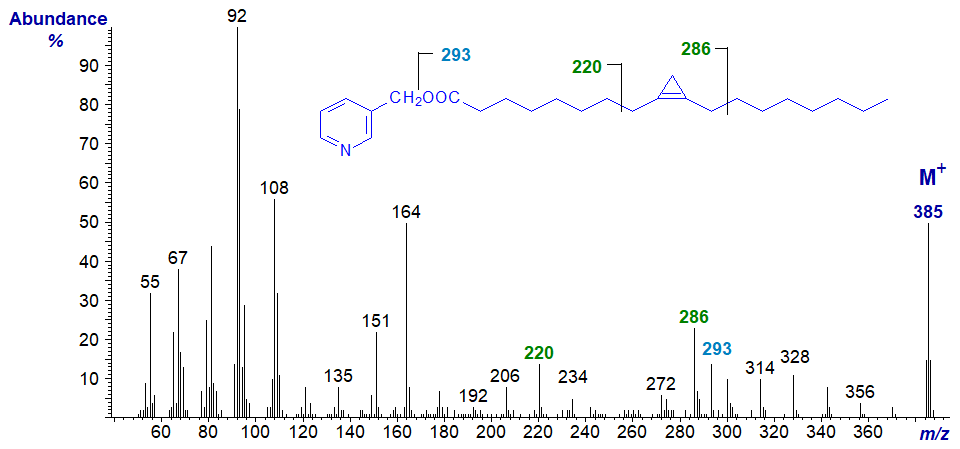
In this instance, the diagnostic cleavages occur on either side of the ring and beta to it, giving distinctive ions at m/z = 220 and 286. There is a further characteristic ion at m/z = 293 (though not found apparently by Spitzer, V. et al, 1994), which has now been identified as containing the fatty acyl chain without the 3‑pyridylcarbinyl moiety, i.e., it represents a distinctive type of fragmentation that appears to be unique to and diagnostic for cyclopropene rings (Knothe, G. et al., 2011).
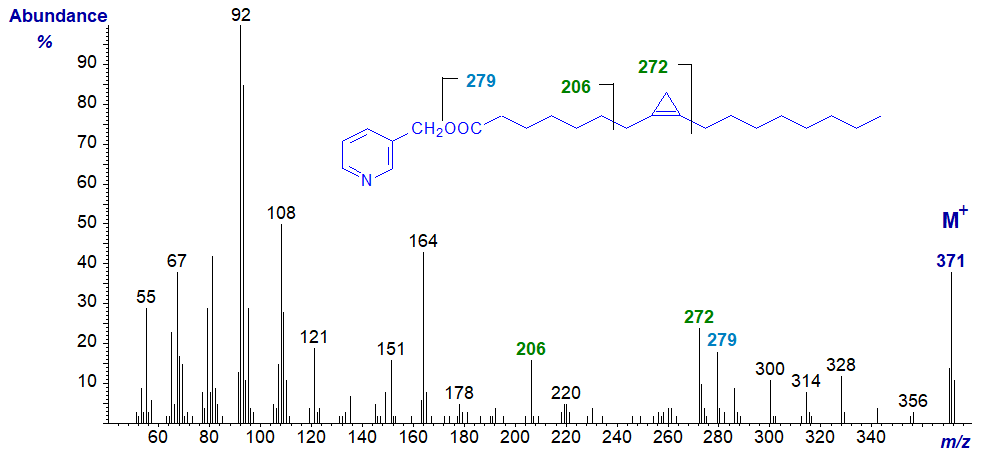
As might be expected, the analogous diagnostic ions in the mass spectrum of 3-pyridylcarbinyl malvalate (8,9-methylene-heptadec-8-enoate) are all 14 amu lower.
ω-Cyclopentenyl and Cyclopentyl Fatty Acids
Fatty acids with a terminal cyclopent-2-enyl moiety are found in high concentration in the seed oils of several species from the plant family Flacourtiaceae, though the corresponding cyclopentyl (saturated species) have been detected at low levels only.
In my opinion, 3-pyridylcarbinol esters are by far the best for characterization of fatty acids with cyclopentenyl and cyclopentyl moieties, especially when these are on the terminal or ω-carbon of the aliphatic chain (Christie, W.W. et al., 1989), and the mass spectrum of 3‑pyridylcarbinyl 11‑cyclopentylundecanoate is illustrated next -
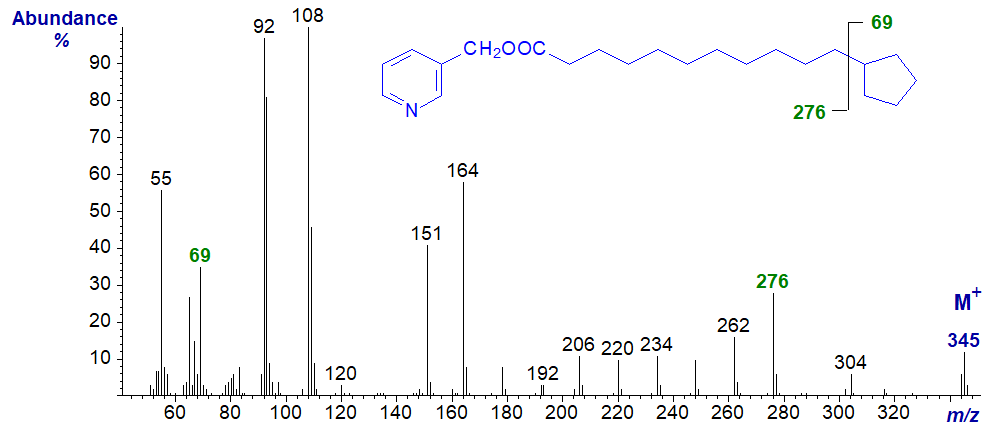
In the high mass region, the molecular ion (at m/z = 345) is followed by a relatively clear gap of 69 amu to an ion at m/z = 276, representing loss of the cyclopentane ring. Thereafter, there is a regular series of ions 14 amu part for cleavage at the successive methylene groups. The ion for the charged cyclopentane ring (m/z = 69) is also distinctive.
3-Pyridylcarbinol esters are especially useful with cyclopentenyl fatty acids, as illustrated with the mass spectrum of 3-pyridylcarbinyl hydnocarpate (11‑cyclopentenylundecanoate) -
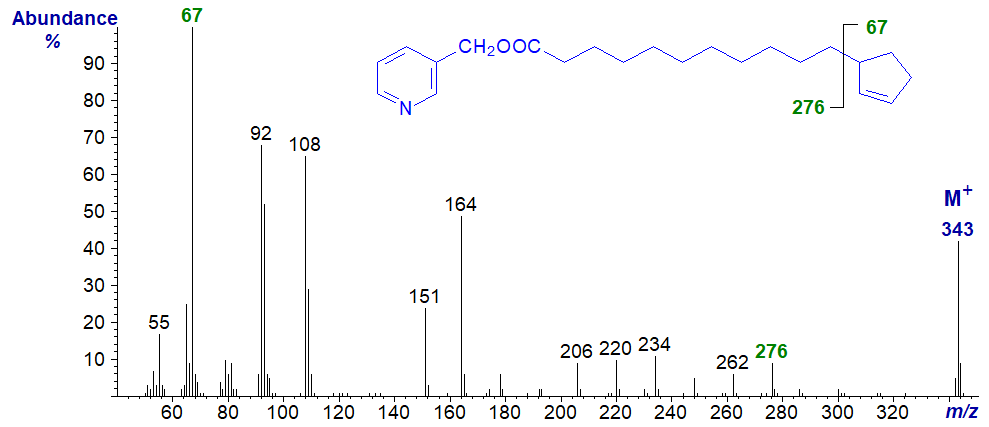
Here the gap of 67 amu between the molecular ion and that at m/z = 276 is clearly diagnostic for loss of the cyclopentene ring, while the ionized cyclopentene ring (m/z = 67) is in fact the base ion.
Three main isomers with one double bond in the chain are found in nature and the only isomer where identification might be problematic is 3‑pyridylcarbinyl 13‑cyclopent-2-enyltridec-4-enoate -
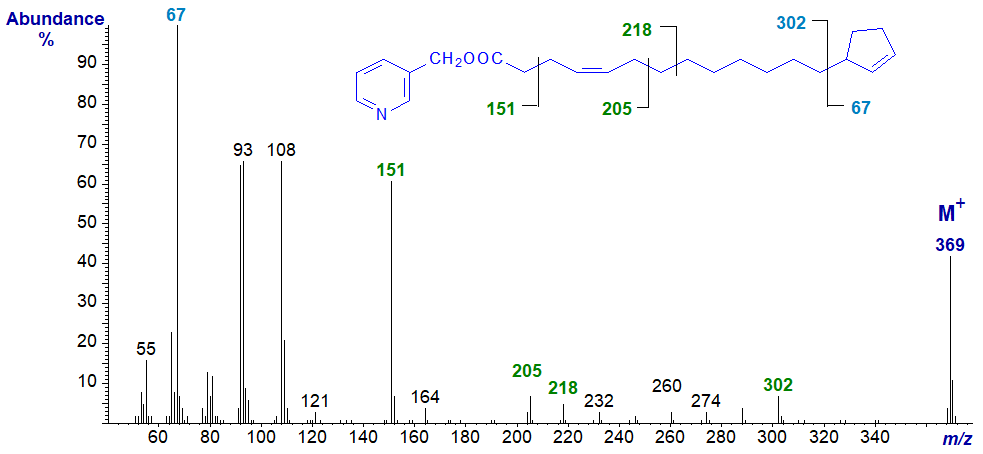
The cyclopentene ring is located by the gap of 67 amu in the high mass region of the spectrum as before (with the ion at m/z = 67 as the base ion). The double bond in position 4 is recognized by the fingerprint ions at m/z = 205 and 218, together with the ion at m/z = 164 of low intensity relative to that at m/z = 151 (see the original reference (Christie, W.W. et al., 1989) or the section of the mass spectrometry pages on 3‑pyridylcarbinol esters of monoenes. There is obviously no double bond between carbons 6 and 13 as can be determined from the regular series of ions 14 amu apart from m/z = 218 to 302.
Similarly, with 3-pyridylcarbinyl 15-cyclopent-2-enylpentadec-9-enoate or hormelate (next spectrum), the ions for the cyclopentene ring and its loss are highly characteristic, and the position of the double bond in position 9 can be stated with absolute certainty from a knowledge of the spectra of the appropriate monoene standards.
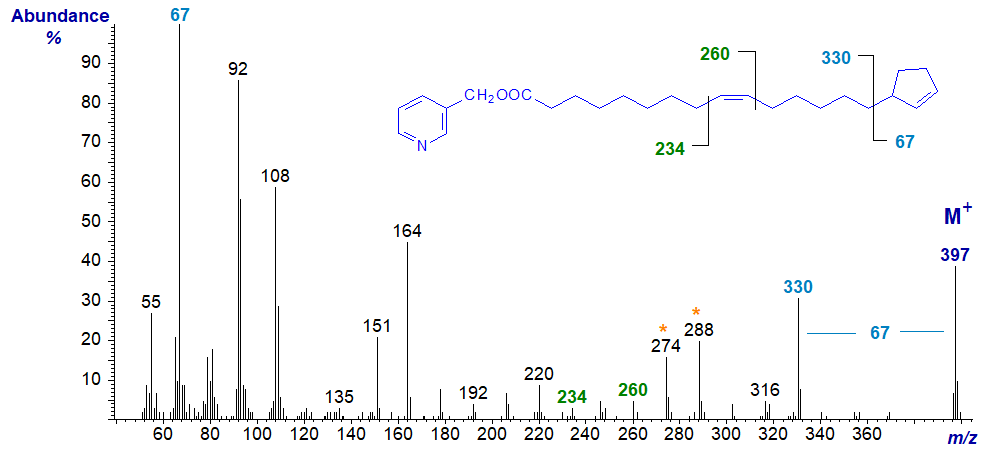
ω-Phenyl Fatty Acids
Fatty acids with a terminal phenyl moiety are found in seed oils of the subfamily Aroideae of the Araceae and certain species of bacteria. Some of the available data have been published (Christie, W.W., 2003).
3-Pyridylcarbinyl 13-phenyl-tridecanoate has the mass spectrum illustrated next. The distinctive and diagnostic feature is a gap of 91 amu between m/z = 290 and 381 (M+) for loss of the terminal phenyl group together with carbon-13, presumably as the stable tropylium ion (see our web page on the methyl ester derivatives of cyclic fatty acids for a discussion). Thereafter, there are sequential gaps of 14 amu for loss of successive methylene groups in the aliphatic chain. The tropylium ion per se is the base ion.
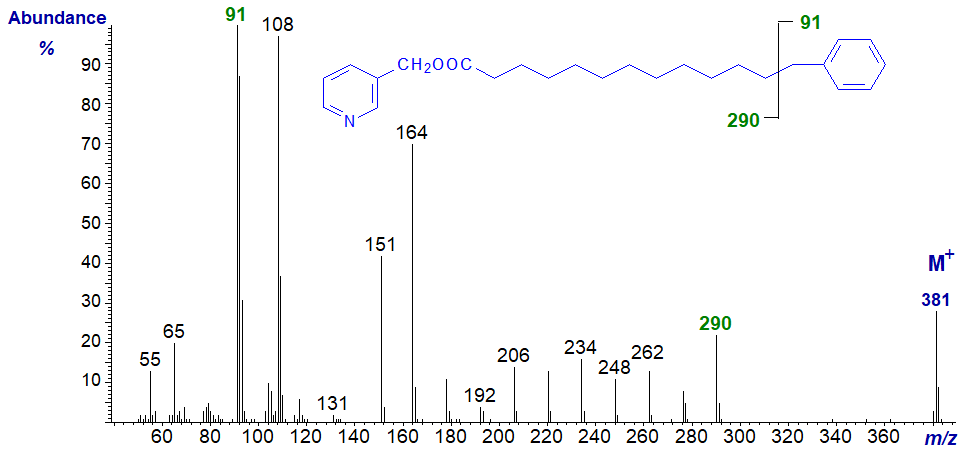
Spectra of further homologues have now been published (Schröder, M. et al., 2014).
When there is a further double bond in the alkyl chain, it can be located in a relatively straight-forward manner with 3-pyridylcarbinol esters. For example, the mass spectrum of 3-pyridylcarbinyl 15-phenyl-pentadec-9-enoate is –
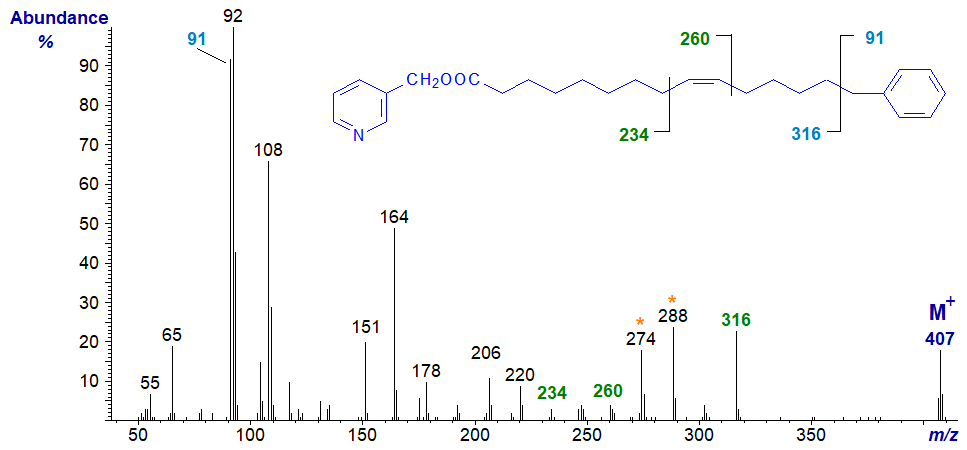
In addition to the ions that locate the phenyl moiety, the position of the double bond in the alkyl chain is easily determined from the ions highlighted (see our web page on the 3-pyridylcarbinol derivatives of monoenes for a detailed explanation, as for that of the hormelate derivative above).
There are mass spectra of 3-pyridylcarbinol esters of many more cyclic fatty acids in our Archive section, but without interpretation.
Cyclic Fatty Acids Formed in Frying Oils
Fatty acids with internal five- and six-membered ring structures are formed when vegetable oils are heated to the high temperatures attained during frying or the physical refining process. The mechanism of cyclization is now believed to involve concerted thermal rearrangements with loss of one of the double bonds. As a host of basic structures can be formed from different fatty acid precursors, and cis-trans isomerization of double bonds and/or about the ring takes place, the range of cyclic products is very great. With my colleagues and collaborators, we have characterized most of these with the aid of both 3-pyridylcarbinol esters and dimethyloxazoline derivatives, and of course others have worked on the problem (for a review see Christie, W.W. and Dobson, G., 2000).
This is too specialized a topic for detailed discussion here, but I illustrate two spectra from cyclic fatty acids from heated soybean oil after hydrogenation to show how centrally positioned ring structures can be located. Note that the points of attachment of the substituents to the rings are not known with certainty.
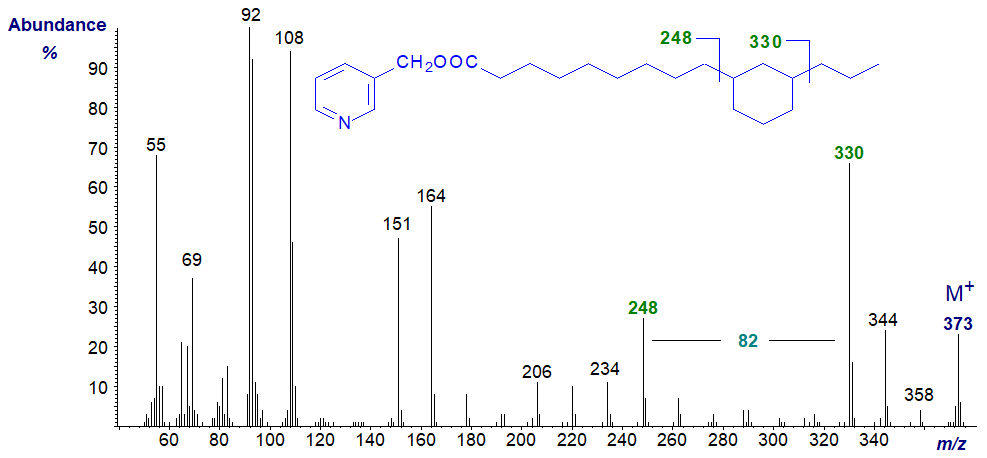
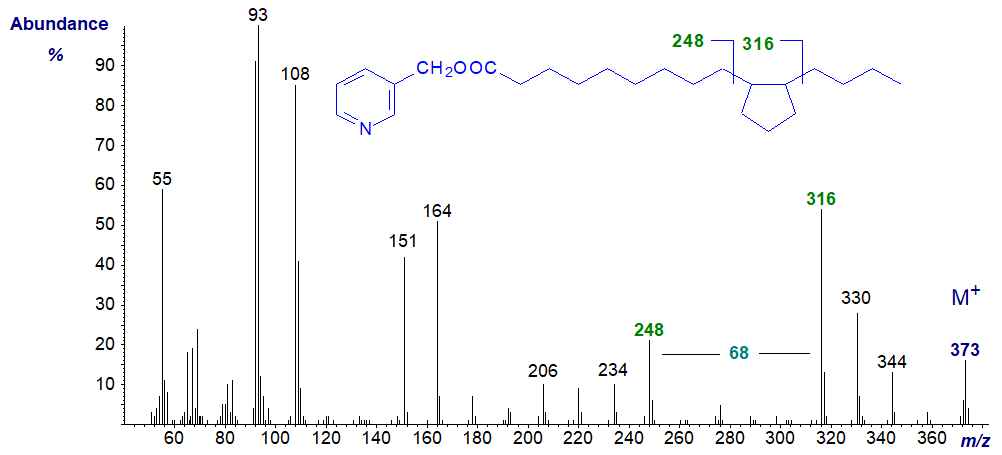
References
- Christie, W.W. 13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in Arum maculatum seed oil. Eur. J. Lipid Sci. Technol., 105, 779-780 (2003); DOI.
- Christie, W.W. and Dobson, G. Formation of cyclic fatty acids during the frying process. Eur. J. Lipid Sci. Technol., 102, 515-520 (2000); DOI.
- Christie, W.W., Brechany, E.Y. and Shukla, V.K.S. Analysis of seed oils containing cyclopentenyl fatty acids by combined chromatographic procedures. Lipids, 24, 116-120 (1989); DOI.
- Harvey, D.J. Picolinyl derivatives for the characterization of cyclopropane fatty acids by mass spectrometry. Biomed. Environm. Mass Spectrom., 11, 187-192 (1984); DOI.
- Knothe, G., Rashid, U., Yusup, S. and Anwar, F. Fatty acids of Thespesia populnea: mass spectrometry of picolinyl esters of cyclopropene fatty acids. Eur. J. Lipid Sci. Technol., 113, 980-984 (2011); DOI.
- Sébédio, J.L. and Grandgirard, A. Cyclic fatty acids: natural sources, formation during heat treatment, synthesis and biological properties. Prog. Lipid Res., 28, 303-336 (1989); DOI.
- Schröder, M., Abdurahman, H., Ruoff, T., Lehnert, K. and Vetter, W. Identification of aromatic fatty acids in butter fat. J. Am. Oil Chem. Soc., 91, 1695-1702 (2014); DOI.
- Spitzer, V., Marx, F., Maia, J.G.S. and Pfeilsticker, K. The mass spectra of the picolinyl ester derivatives of malvalic and sterculic acid. Fat Sci. Technol., 96, 395-396 (1994); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 17th, 2023 | Contact/credits/disclaimer | |
