Mass Spectrometry of 3-Pyridylcarbinol Esters
Acetylenic Fatty Acids
 As
with other documents in this section, this is a subjective account of mass spectrometry with electron-impact ionization
of acetylenic fatty acids in the form of the 3-pyridylcarbinol ('picolinyl') esters,
detailing only those encountered during our research activities here and for which we have spectra available for illustration purposes,
although I trust that we have a wider range of spectra than are likely to be encountered elsewhere.
Many have never been published formally, but I cite references to prior publications when these are known to us.
Spectra of methyl esters and of
DMOX/pyrrolidide derivatives of acetylenic fatty acids are described in separate web pages.
Spectra of highly unsaturated acetylenic fatty acids are rarely easy to interpret,
and a common failing is to attempt to see more in a spectrum than may be justified.
It is often useful to simply treat a mass spectrum as a 'fingerprint' for comparison purposes when standards are available.
Another potential problem is that such fatty acids can isomerize and oxidize readily on derivatization or if handled carelessly.
As with our other web pages, this is not intended as a mechanistic guide, and fragmentations are depicted simplistically.
The chemistry and occurrence of natural acetylenic fatty acids is discussed
in the Lipid Essentials section of this website.
As
with other documents in this section, this is a subjective account of mass spectrometry with electron-impact ionization
of acetylenic fatty acids in the form of the 3-pyridylcarbinol ('picolinyl') esters,
detailing only those encountered during our research activities here and for which we have spectra available for illustration purposes,
although I trust that we have a wider range of spectra than are likely to be encountered elsewhere.
Many have never been published formally, but I cite references to prior publications when these are known to us.
Spectra of methyl esters and of
DMOX/pyrrolidide derivatives of acetylenic fatty acids are described in separate web pages.
Spectra of highly unsaturated acetylenic fatty acids are rarely easy to interpret,
and a common failing is to attempt to see more in a spectrum than may be justified.
It is often useful to simply treat a mass spectrum as a 'fingerprint' for comparison purposes when standards are available.
Another potential problem is that such fatty acids can isomerize and oxidize readily on derivatization or if handled carelessly.
As with our other web pages, this is not intended as a mechanistic guide, and fragmentations are depicted simplistically.
The chemistry and occurrence of natural acetylenic fatty acids is discussed
in the Lipid Essentials section of this website.
Monoynoic Fatty Acids
Octadec-9-ynoic or stearolic acid is a minor component of certain seed oils, although it has long been known as a synthetic product. The mass spectrum of the 3‑pyridylcarbinol ester of octadec-9-ynoate has features that serve to locate the triple bond. It should be compared with that of 3‑pyridylcarbinol oleate, which it resembles superficially -
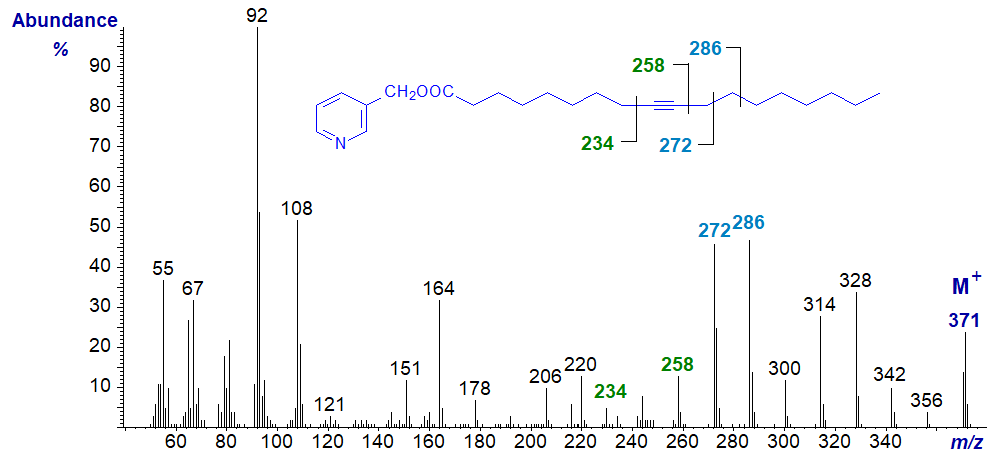
The ions at m/z = 92, 108, 151 and 164 are characteristic of the 3-pyridylcarbinol moiety (see our web page on the analogous saturated derivatives). The molecular ion and others in the high mass range are of course 2 amu less than with oleate. The acetylenic bond is located by a gap of 24 amu between m/z = 234 and 258. If this is less than convincing, the gap of 38 amu for the triple bond with the proximal methylene between m/z = 220 and 258 is clear, and the two ions at m/z = 272 and 286 (resembling those in the mass spectrum of 3-pyridylcarbinol oleate) are useful signposts.
Diynoic Fatty Acids
We had access some years ago to a comprehensive series of bis-methylene-interrupted and conjugated diynoic fatty acids prepared by M.S.F. Lie Ken Jie and colleagues from Hong Kong, and details of the mass spectra of the 3-pyridylcarbinol ester derivatives (only) were published (Christie et al., 1988). For most scientists, these are likely to be of limited academic interest, so a few representatives only are shown below. Spectra of polyacetylenic fatty acids are rarely easy to interpret, as triple bonds rearrange in complex ways under electron bombardment.
Mass spectrum of 3-pyridylcarbinyl 5,9-octadecadiynoate -
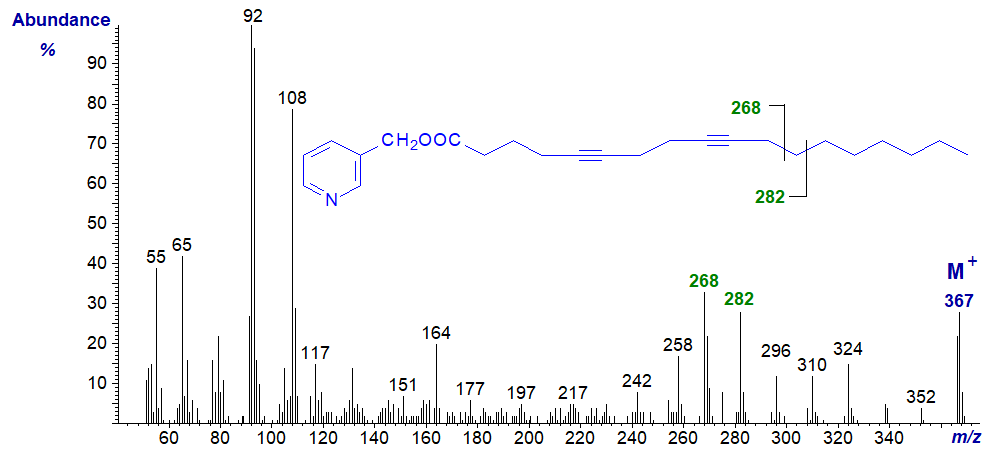
To someone who did not know the structure, it would be evident that there were eight hydrogens fewer than those present in a saturated compound, and that the deficiency must be before carbon-11. Otherwise, the spectrum can only be useful as a fingerprint for comparison purposes. However, the ion formed by fragmentation beta to the final double or triple bond is distinctive here and in most of the spectra that follow.
Mass spectrum of 3-pyridylcarbinyl 9,13-octadecadiynoate
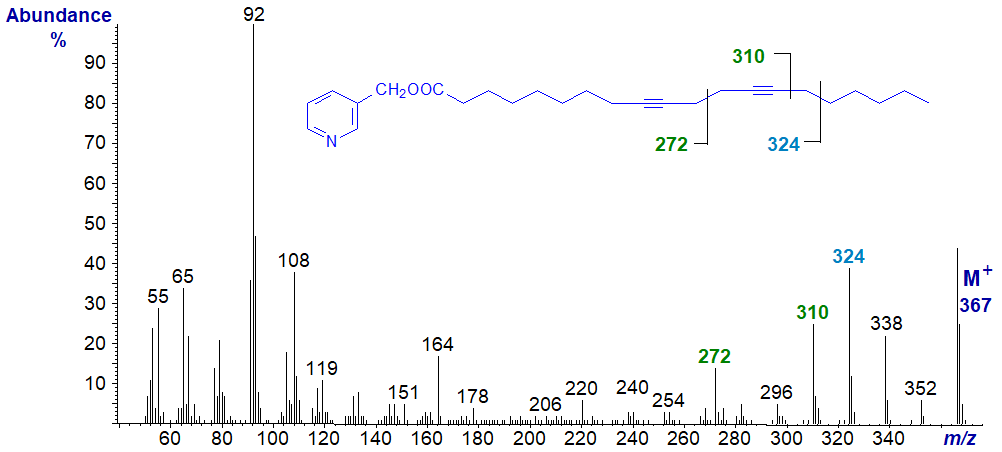
Here, the triple bond in position 13 can be located from the gap of 38 amu between m/z = 272 and 310, but that in position 9 cannot be located definitively. In this and many of the other related spectra, the ion for [M−1]+ is more abundant than the molecular ion.
The mass spectrum of the conjugated diyne 3-pyridylcarbinyl 9,11-octadecadiynoate is -
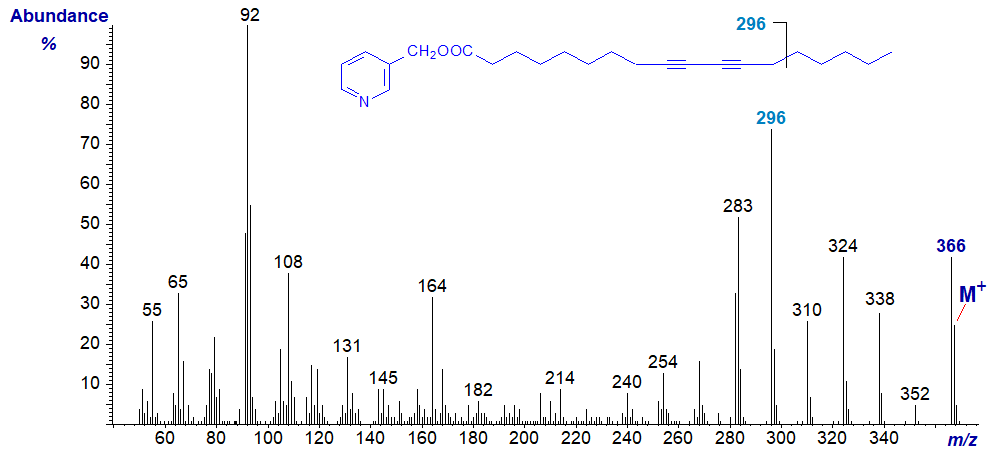
Spectra of other isomers with triple bonds in relatively central positions are all very similar to this (see the Archive section), so I suspect complex rearrangements occur internally under electron bombardment to smooth out potential differences. Therefore, no interpretation of the spectrum is offered.
Methylene-Interrupted Ene-ynoic Fatty Acids
Crepenynic or octadec-9-en-12-ynoic acid is a major constituent of some seed oils and is important as a biosynthetic precursor of a family of secondary metabolites in plants. 3-Pyridylcarbinyl crepenynate has a relatively informative spectrum (Christie, 1998) -
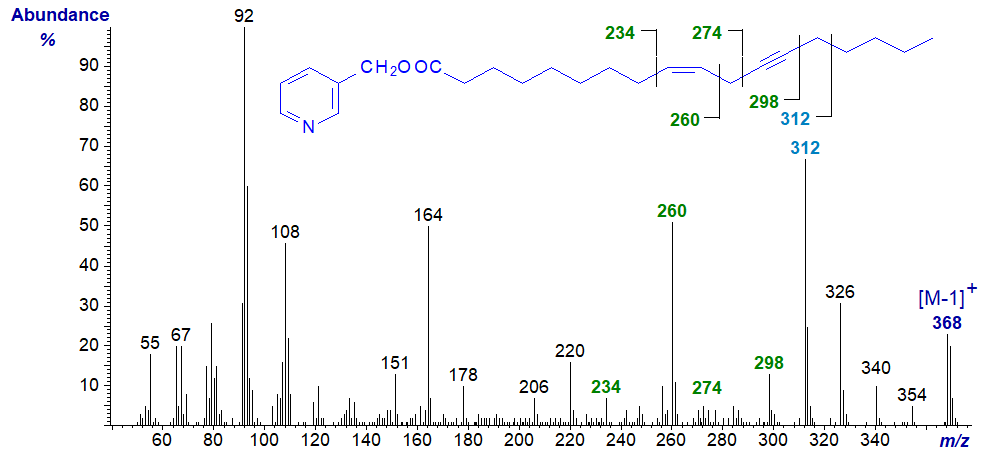
In all the relevant spectra available to us, the ion representing [M−1]+ is more abundant than the molecular ion. In this instance, interpretation is straightforward, as the double bond is recognized by the gap of 26 amu between m/z = 234 and 260, as in the spectrum of 3‑pyridylcarbinol oleate, for example, while the triple bond is characterized by the gap of 24 amu between m/z = 274 and 298, or better by the gap of 38 amu between m/z = 260 and 298. The prominent ions at m/z = 312 and 326 are also of diagnostic value.
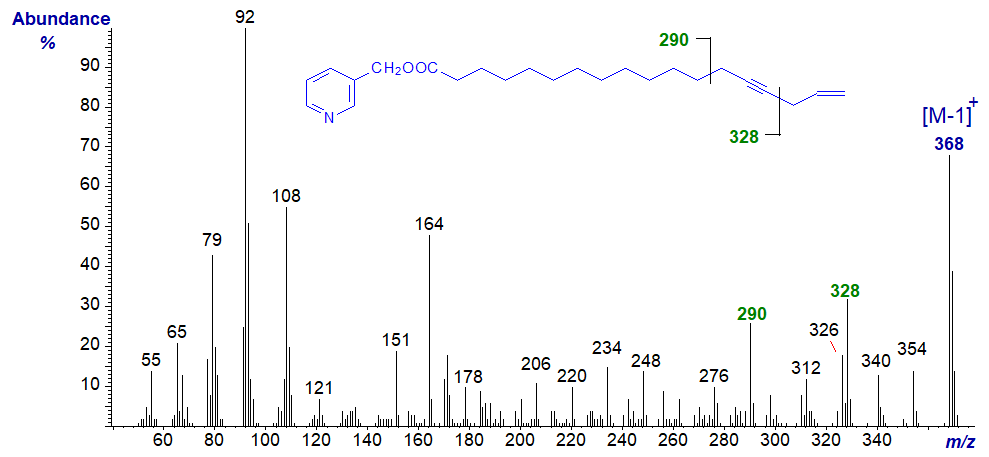
In the spectrum of the C20 analogue, 3-pyridylcarbinyl eicos-11-en,14-ynoate, obtained by incubation of Spirulina platensis with crepenynic acid, the equivalent diagnostic ions are 28 amu higher as expected and indicated on the figure.
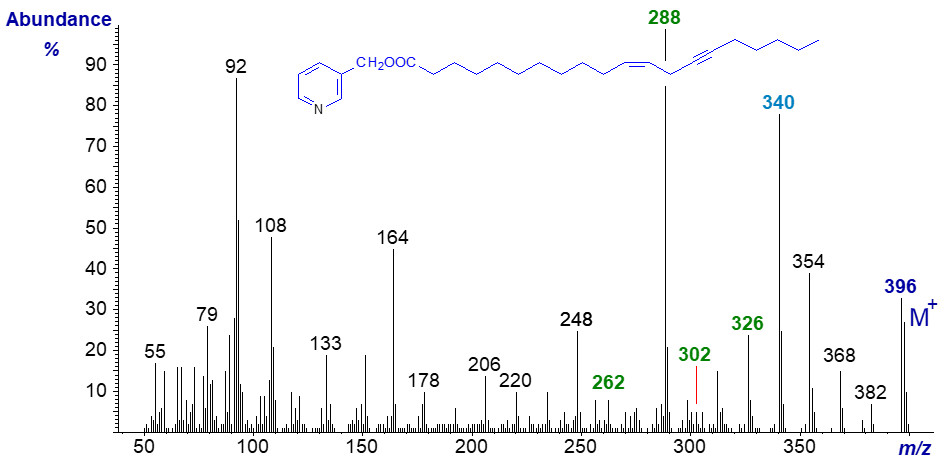
The mass spectrum of 3-pyridylcarbinyl octadeca-14-yn,17-enoate, a synthetic product (published in Christie (1998)) -
Again, there is room for confusion. A gap of 38 amu between ions at m/z = 290 and 328 serves to locate the triple bond, and a gap of 41 amu between m/z = 328 and 369 could be construed as indicative of a terminal double bond, although an expected ion at m/z = 304 for cleavage between carbons 13 and 14 is not apparent. It is noteworthy that the spectrum resembles that of 14,17-octadecadienoate (Christie et al., 1987 or see the web page on 3‑pyridylcarbinol esters of methylene-interrupted dienes). From the low molecular weight end of the spectrum to m/z = 290, they are virtually identical, but from then on that of the ene-yne ester has comparable prominent ions but 2 amu lower.
The 3-pyridylcarbinol ester of octadeca-6,9-dien-12-ynoate, produced from crepenynic acid by a Δ6 desaturase on incubation with the cyanobacterium Spirulina platensis (author, unpublished), has the spectrum -
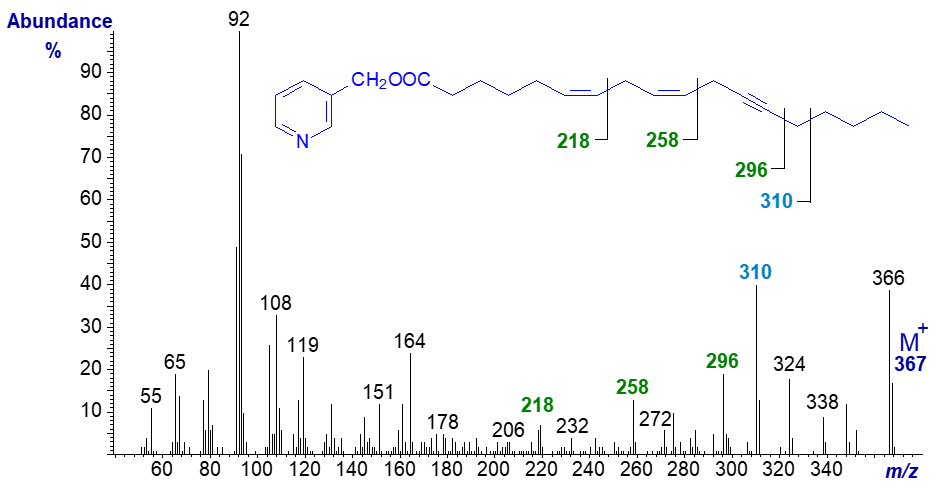
The triple bond in position 12 is most easily recognized by the gap of 38 amu between m/z = 258 and 296, while the double bond in position 9 is located by the gap of 40 amu between m/z = 218 and 258. The position of the double bond in position 6 must be inferred from the fact that if it was in any other position, substantial changes to the spectrum would be expected. For example, if the double bond was in position 5, a prominent ion at m/z = 219 would be predicted (see the web page on 3-pyridylcarbinol esters of bis-methylene-interrupted dienes).
The 3-pyridylcarbinol ester of octadeca-6-yn,9,12,15-trienoate from the moss Dicranum scoparium (courtesy of Gary Dobson) has the spectrum -
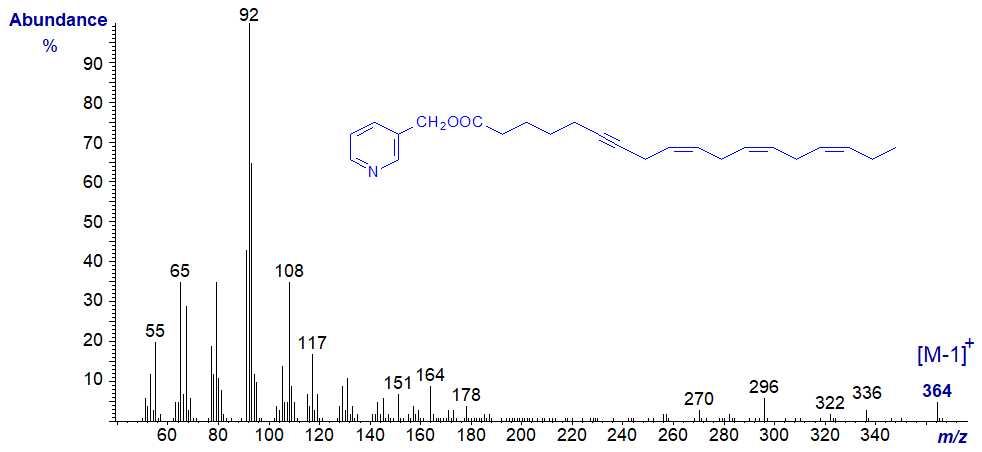
It would be impossible to draw any significant structural information from this spectrum, other than its molecular weight and thence its chain length and degree of unsaturation. The fatty acid was in fact identified by deuteration and conversion to the DMOX derivative, an approach that may be necessary for other polyen/ynoic fatty acids (Guschina et al., 2002).
Conjugated Ene-ynoic Fatty Acids
The 3-pyridylcarbinol ester of ximenynic or santalbic or octadec-9-yn,11-trans-enoic acid, a component of certain seed oils, has the mass spectrum -
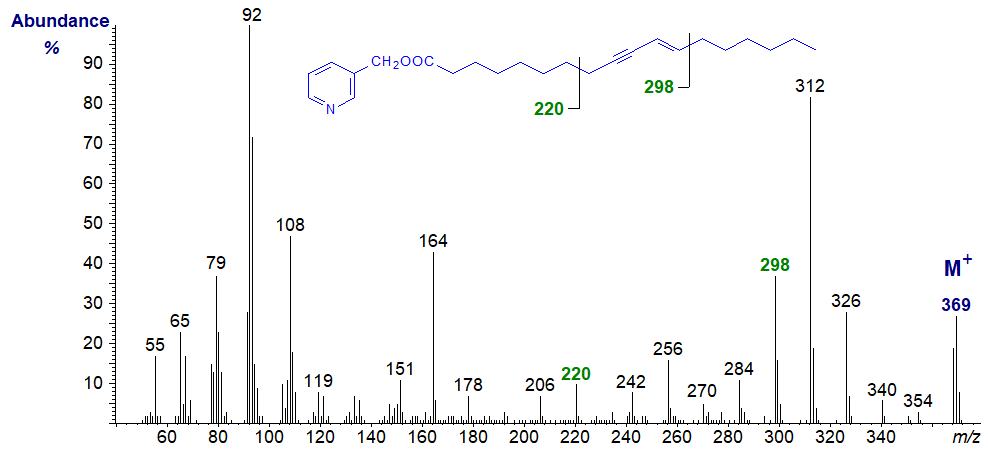
It is evident from this that the unsaturation (loss of six H) must be somewhere between carbons 8 and 13 (m/z = 220 and 298), but other than this it would not be easy to locate the double and triple bonds more specifically, possibly because complex rearrangements occur either during preparation of the derivative, or at the high temperature required for gas chromatography or as part of the electron-impact ionization. The spectrum is best considered as a fingerprint, therefore.
The spectrum of the homologous 3-pyridylcarbinyl eicos-11-yn,13-trans-enoate exhibits many comparable features, but shifted consistently 28 amu upwards -
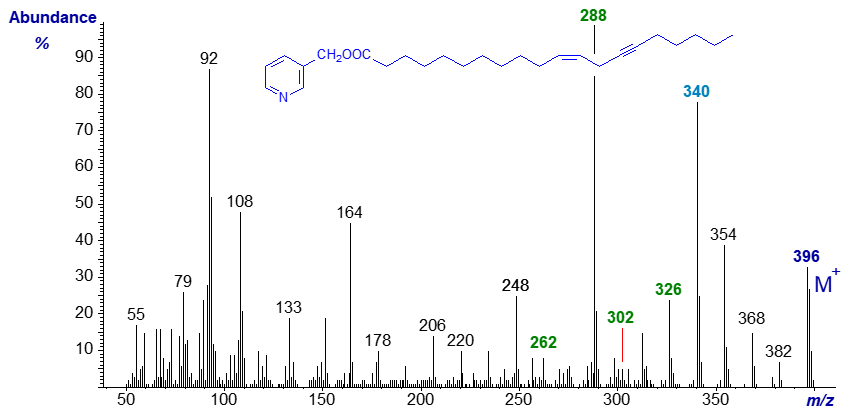
We have spectra 3-pyridylcarbinol esters of other homologues of these fatty acids up to C28 in chain-length in our Archive section (and of many more acetylenic fatty acids).
With the mass spectrum of 3-pyridylcarbinyl octadeca-8,10-dien-12-ynoate, from the seed oil of Tanacetum corymbosum, the picture is even more complicated -
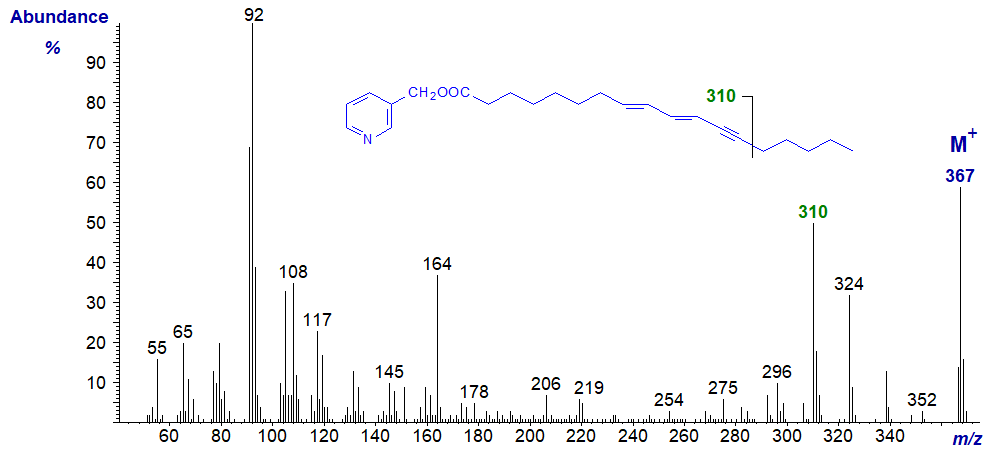
Although the ion for fragmentation beta to the terminal triple bond is again distinctive at m/z = 310, it was only possible to identify the location of the double and triple bonds by isolating the fatty acid and performing deuteration prior to mass spectrometry of the 3‑pyridylcarbinol ester (Tsevegsuren et al., 1988). See the section of this website on 'Mass spectra of methyl esters of fatty acids - further derivatization' for a detailed protocol and additional information.
References
- Christie, W.W. Mass spectrometry of fatty acids with methylene-interrupted ene-yne systems. Chem. Phys. Lipids, 94, 35-41 (1998); DOI.
- Christie, W.W., Brechany, E.Y. and Holman, R.T. Mass spectra of the picolinyl esters of isomeric mono- and dienoic fatty acids. Lipids, 22, 224-228 (1987); DOI.
- Christie, W.W., Brechany, E.Y. and Lie Ken Jie, M.S.F. Mass spectra of the picolinyl ester derivatives of some isomeric dimethylene-interrupted octadecadiynoic acids. Chem. Phys. Lipids, 46, 225-229 (1988); DOI.
- Guschina, I.A., Dobson, G. and Harwood, J.L. Lipid metabolism in the moss Dicranum scoparium: effect of light conditions and heavy metals on the accumulation of acetylenic triacylglycerols. Physiologia Plantarum, 116, 441-450 (2002); DOI.
- Tsevegsuren, N., Christie, W.W. and Lösel, D. Tanacetum (Chrysanthemum) corymbosum seed oil: a rich source of a novel conjugated acetylenic acid. Lipids, 33, 723-727 (1998); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
