Mass Spectrometry of 3-Pyridylcarbinol Esters
Monoenoic Fatty Acids
The mass spectra of 3-pyridylcarbinol ('picolinyl') esters of monoenoic fatty acids are distinctive and permit location of the double bond with relative ease, though access to standard spectra is always helpful, especially when the double bond is close to either end of the molecule. Our web page on mass spectrometry of 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). The C18 monoenoic fatty acid isomers (straight-chain) for this study were synthesised by Gunstone and Ismail (1967) to whom I am grateful for access. Please note that diagrams showing the fragmentation points over-simplify the processes that occur but are helpful for most practical identification purposes.
Straight-Chain Monoenoic Fatty Acids
First, the mass spectrum of 3-pyridylcarbinyl 9-octadecenoate (oleate), the most important monoenoic acid in nature, is illustrated (Harvey, 1982) -
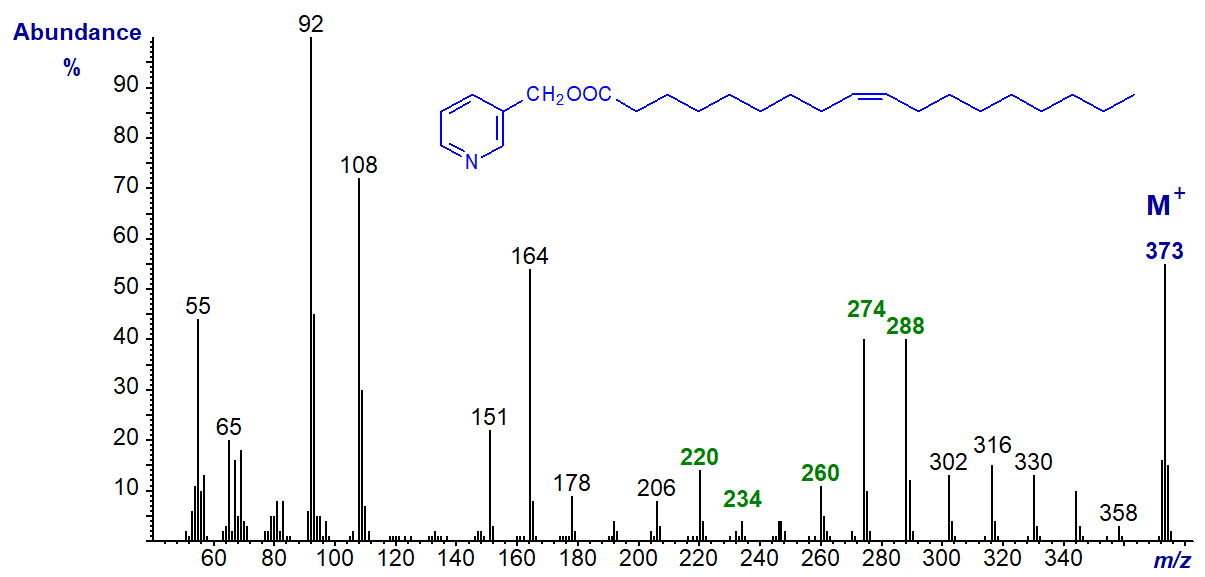
In the low molecular weight region of the spectrum, as with all 3-pyridylcarbinol esters, there are prominent ions at m/z = 92, 108, 151 and 164, which are all fragments about the pyridine ring. Then, the principle of interpretation is the same as that for saturated fatty acids (described here), in that the simplest approach is to start with the molecular ion and progress downwards, as if one were unzipping the molecule one methylene group at a time. Thus, from the molecular ion (m/z = 373), there is loss of a methyl group to m/z = 358, followed by a series of ions 14 amu apart for loss of successive methylene groups, i.e., m/z = 344, 330, 316 and so forth.
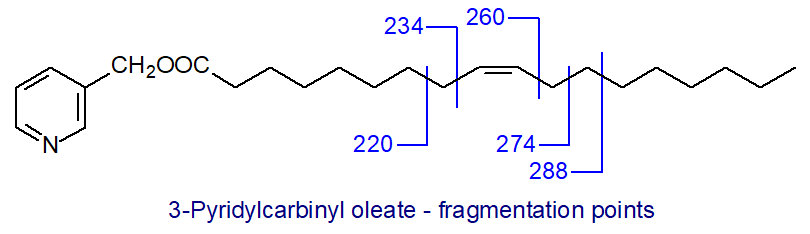
When a double bond is reached, there is a gap of 26 amu between m/z = 234 and 260. This gap can sometimes be difficult to locate precisely, and a fragmentation at the adjacent methylene group on the carboxyl side giving a gap of 40 amu between m/z = 220 and 260 in this instance is often easier to locate, especially with polyenes.
 |
| Diagnostic ions for the double bond in 3-pyridylcarbinol oleate. |
A further distinctive feature of clear diagnostic value is a doublet of abundant ions 14 amu apart, representing cleavage on the distal side of the double bond at m/z = 274 and 288 in this example. In practice, these or the equivalent two ions in other isomers can be picked out for identification purposes even when isomeric fatty acid derivatives are imperfectly resolved by gas chromatography. Formation of the ions has been rationalized in mechanistic terms as an initial abstraction of allylic hydrogen atoms on each side of the double bond with the production of conjugated diene systems, which form relatively stable ions.
The geometry of the double bond makes no readily discernible difference, and the mass spectrum of 3-pyridylcarbinyl elaidate is identical to that of oleate.
Similar series of ions are seen in spectra from 3-pyridylcarbinol esters of most monoenes, but it is advantageous to have access to spectra of authentic standards when the double bond is close to either end of the molecule to avoid any confusion. When interpreting the mass spectra of 3-pyridylcarbinol esters of polyenoic fatty acids, the position of the first double bond is usually hardest to locate from first principles, and a comparison with spectra of standard monoenes can again be useful. Details of the spectra of the complete series of isomeric octadecenoates have been published (Christie, Brechany and Holman, 1987), but only a few of the spectra were depicted in the paper for practical reasons. All are now illustrated below, but please consult the original reference for detailed discussion. Spectra of some homologous fatty acid isomers displaying the same diagnostic features may have been described by others in some instances (not cited).
3-Pyridylcarbinyl 2-octadecenoate (2-18:1) -
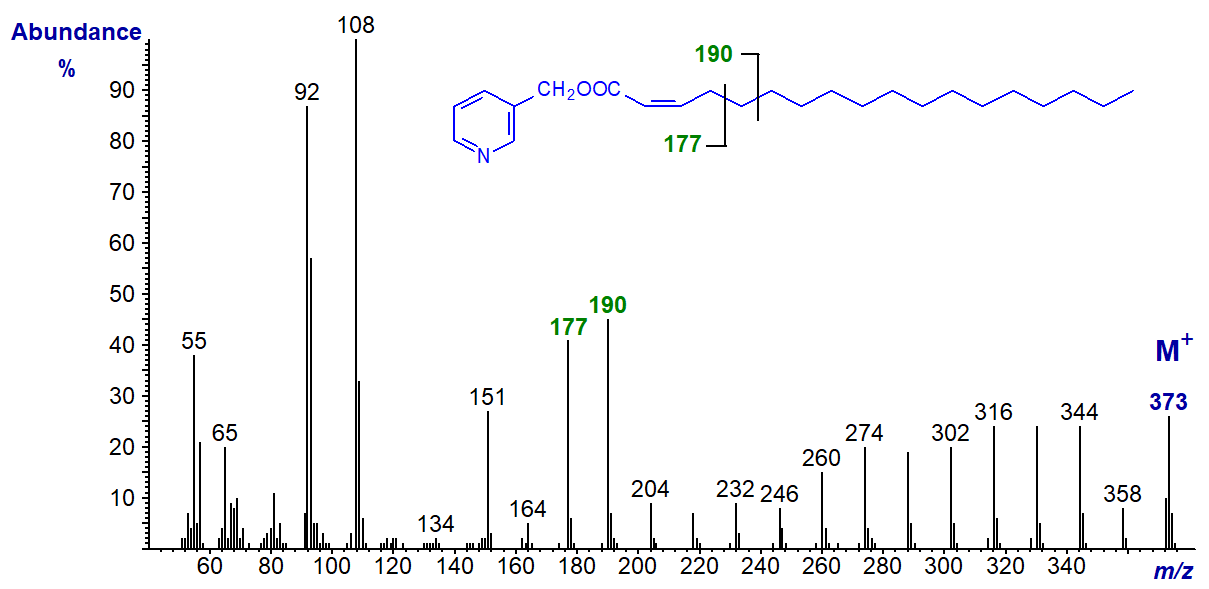
In this mass spectrum, fragments corresponding to cleavage at the double bonds (gaps of 26 amu) are not seen, probably because the double bond forms a stable resonance structure with the carboxyl group. The ion at m/z = 151 is relatively small, and that at m/z = 164, which is usually abundant, is now of low intensity. However, the prominent doublet at m/z = 177 and 190 provides a distinctive fingerprint for identification purposes. The ions regularly spaced 14 amu apart further down the chain confirm that there cannot be a double bond anywhere else in the molecule, and with the next few isomers, the gap of 26 amu for the double bond is not seen but other features can be used for identification.
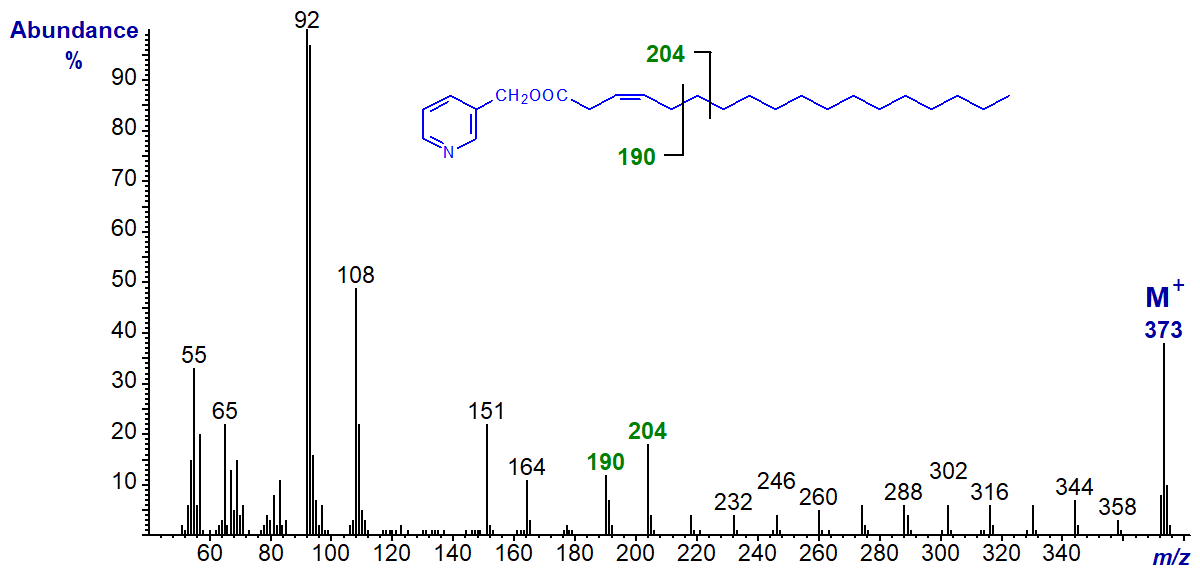
With 3-pyridylcarbinyl 3-octadecenoate (3-18:1) (Christie et al., 1987), the diagnostic doublet has moved by 14 amu to m/z = 190 and 204 -
3-Pyridylcarbinyl 4-octadecenoate (4-18:1). In this instance the double bond in position 4 appears to assist the formation of the ion at m/z = 151, which is the base ion for this isomer only. The relative abundance of the ion at m/z = 218 is also useful for characterization purposes.
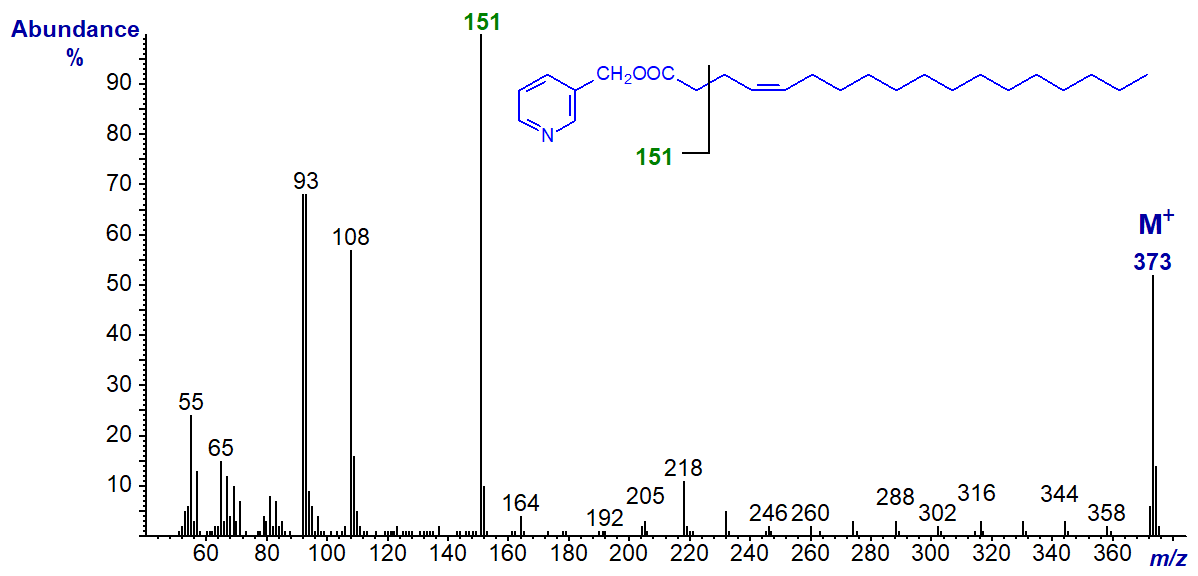
3-Pyridylcarbinyl 5-octadecenoate (5-18:1). Although the gap of 26 amu for the double bond is not easy to locate, that of 40 amu between m/z = 164 and 204 serves instead. Only the second ion of the doublet, at m/z = 232, stands out with this isomer, but this feature appears to be a diagnostic characteristic.
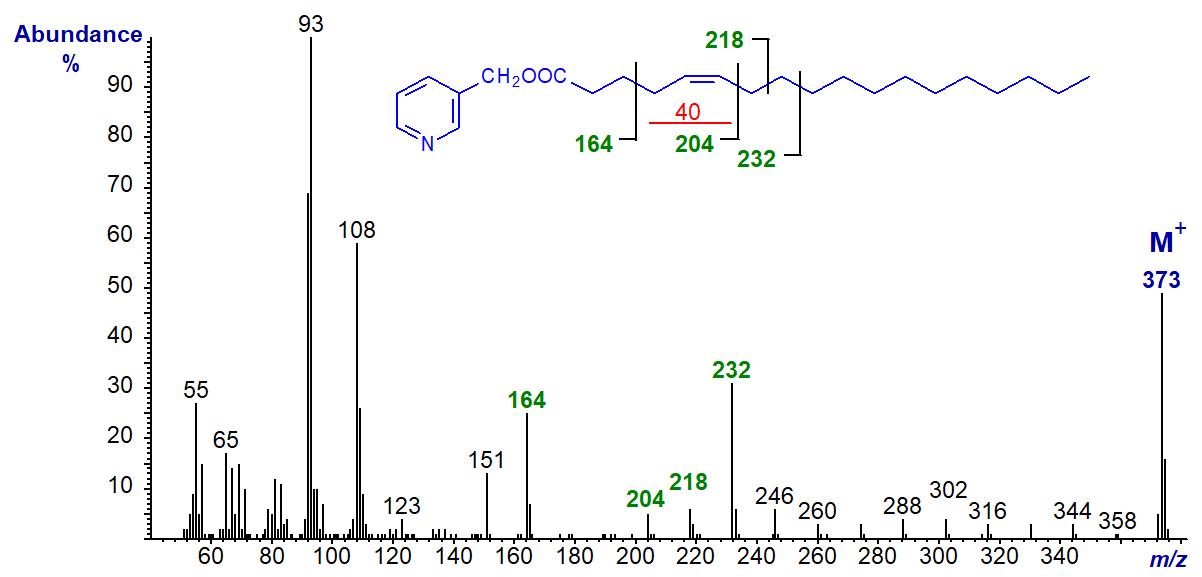
3-Pyridylcarbinyl 6-octadecenoate or petroselinate (6-18:1). This spectrum resembles that of the 5-isomer, except that all the important diagnostic ions are found 14 amu higher.
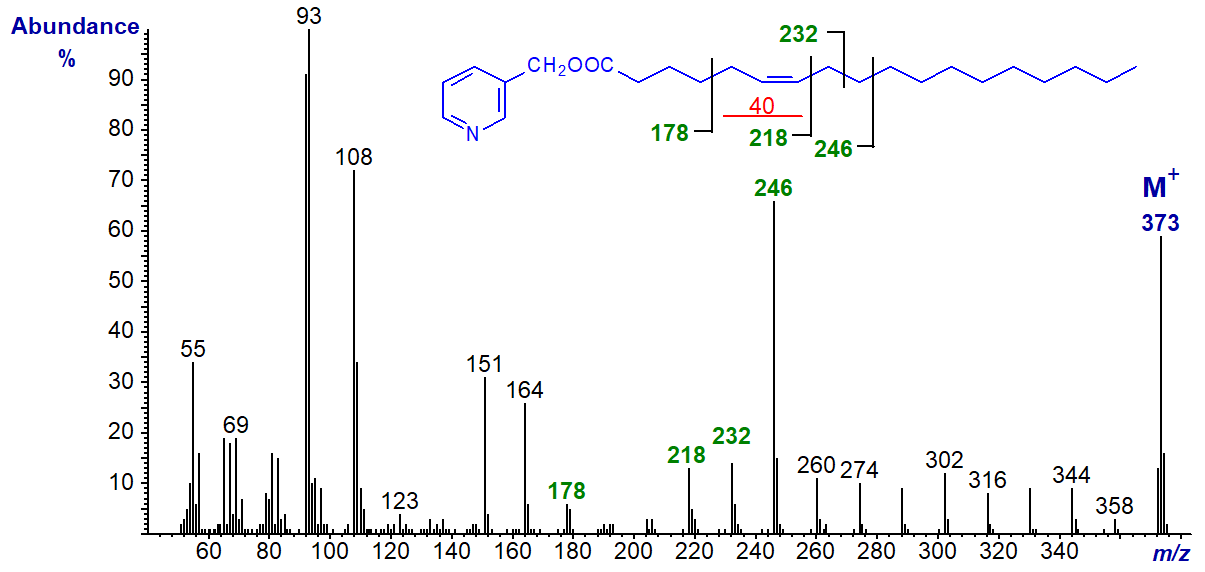
3-Pyridylcarbinyl 7-octadecenoate (7-18:1). From this until the 13-18:1 isomer, the 'typical' pattern for monoenes is observed, i.e., with the gap of 26 amu being clearly apparent, together with the pronounced doublet of ions at higher mass. For the 7-isomer, it is between m/z = 206 and 232, for the 8‑isomer, between 220 and 246, and so on, although the gap of 40 amu is often easier to locate unequivocally.
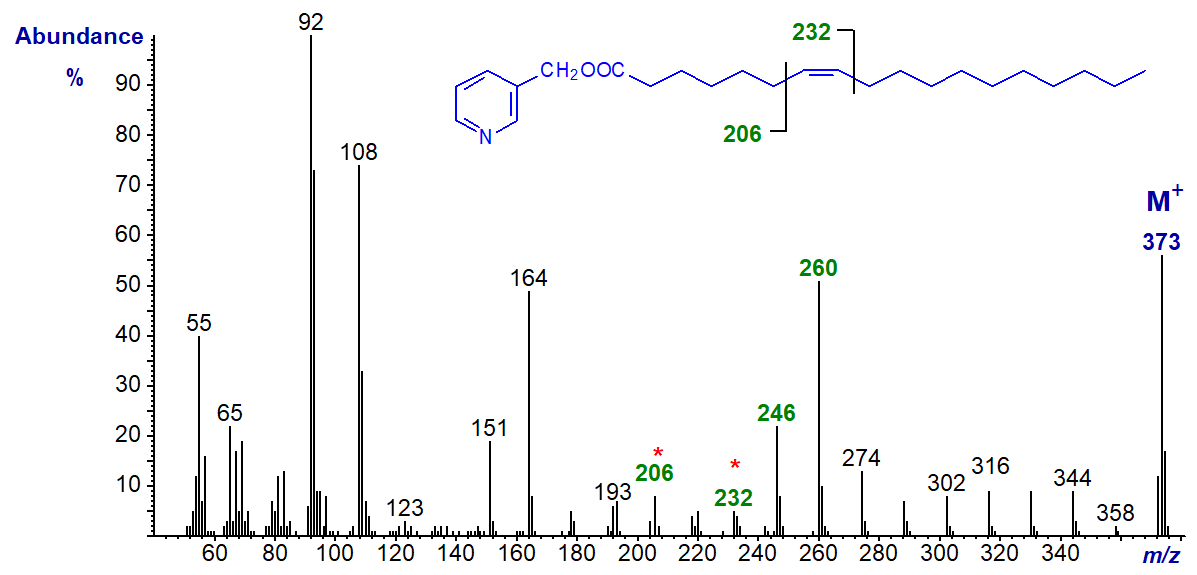
3-Pyridylcarbinyl 8-octadecenoate (8-18:1) -

3-Pyridylcarbinyl 9-octadecenoate (oleate or 9-18:1) - see the first spectrum of this web page. Note that with the later isomers the ion doublets after the double bond become more distinctive until the 14-isomer is reached.
3-Pyridylcarbinyl 10-octadecenoate (10-18:1) (Christie et al., 1987) -
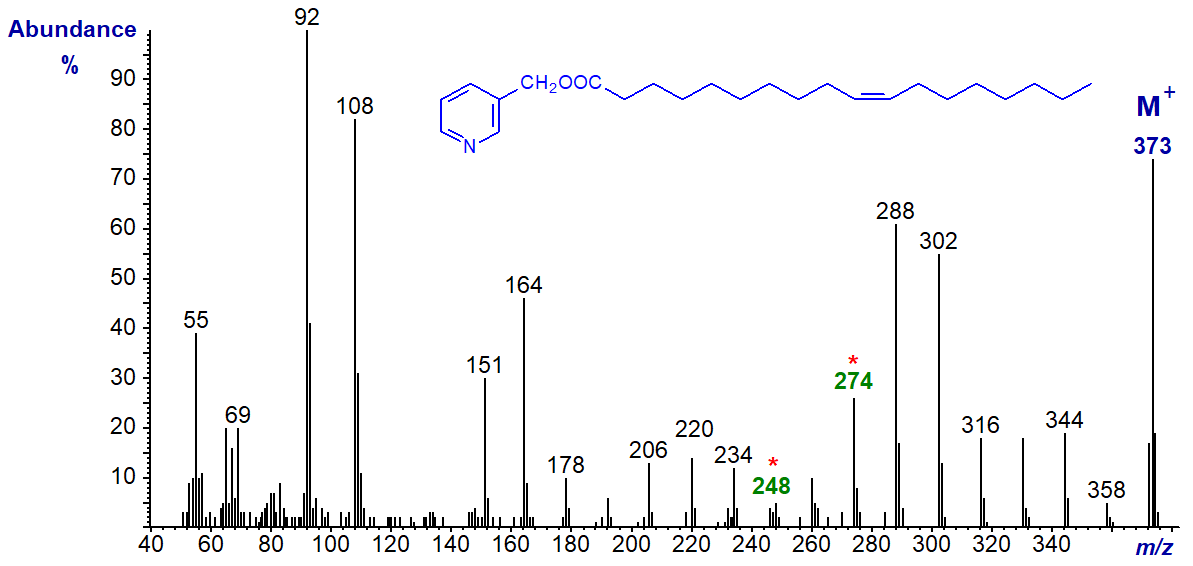
3-Pyridylcarbinyl 11-octadecenoate (cis-vaccenate or 11-18:1) (Harvey, 1982) -
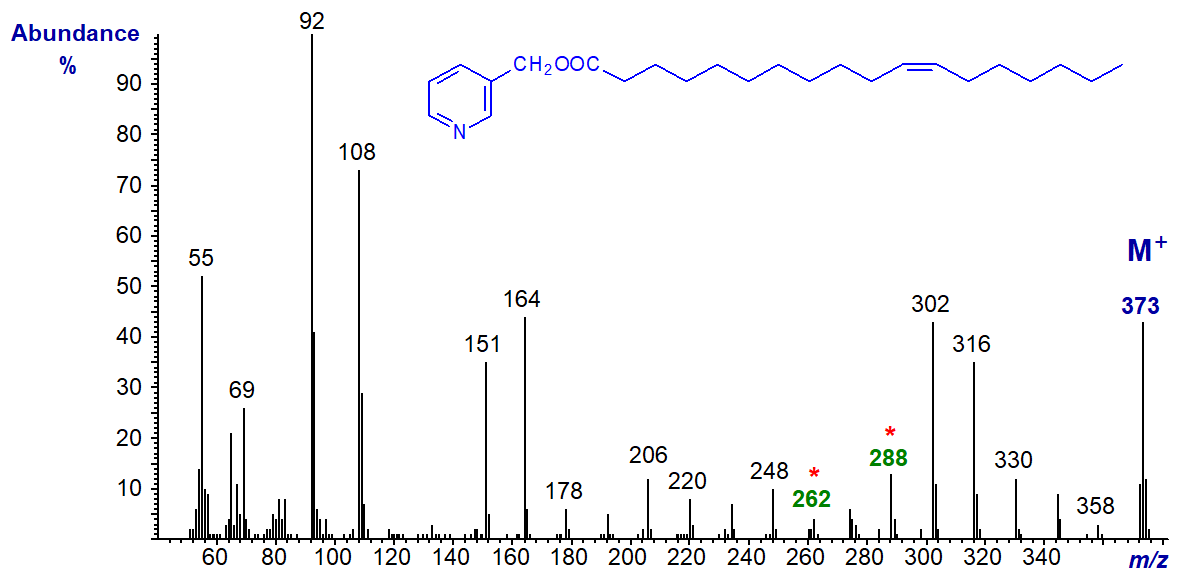
3-Pyridylcarbinyl 12-octadecenoate (12-18:1)
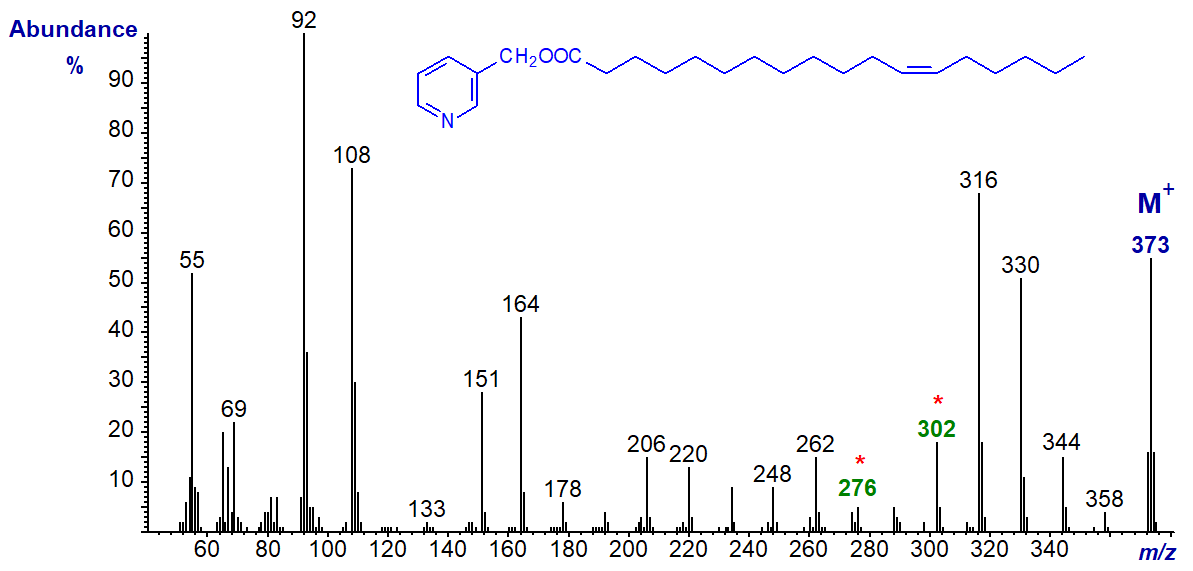
3-Pyridylcarbinyl 13-octadecenoate (13-18:1) -
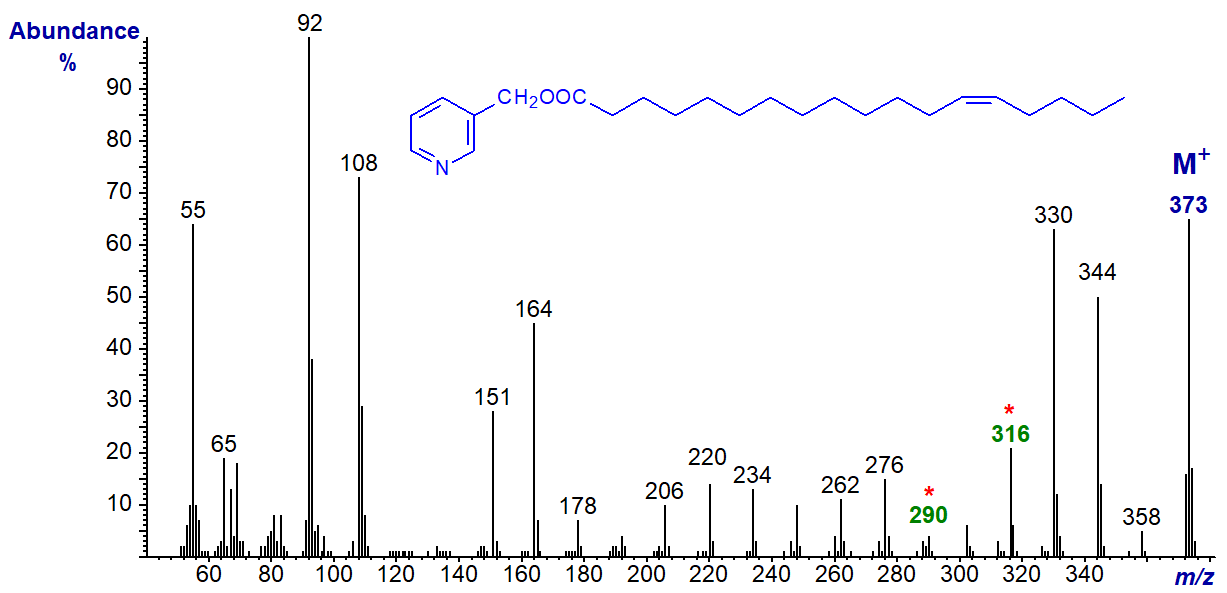
3-Pyridylcarbinyl 14-octadecenoate (14-18:1). As the double bond nears the terminal end of the molecule, the gaps of 26 or 40 amu that locate the double bond are still easy to find, but only the first ion of the expected doublet that should follow (m/z = 344) is now distinctive.
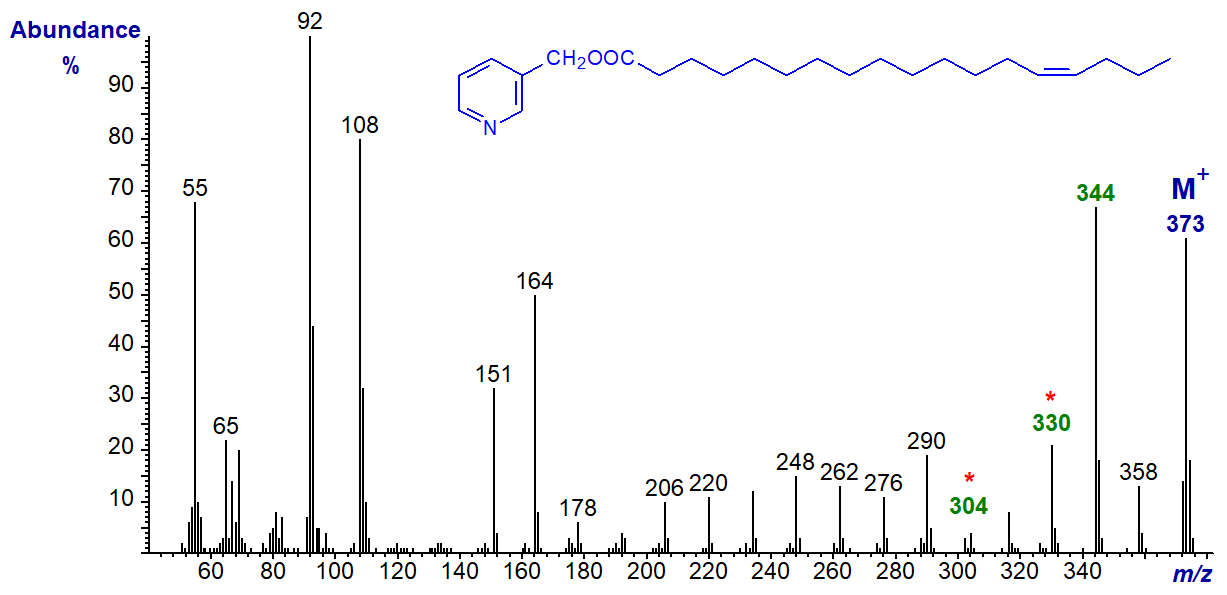
3-Pyridylcarbinyl 15-octadecenoate (15-18:1). Again, the ions characteristic of the double bond are easy to locate, especially the gap of 40 amu, but now the distinctive doublet in the higher mass region is gone -
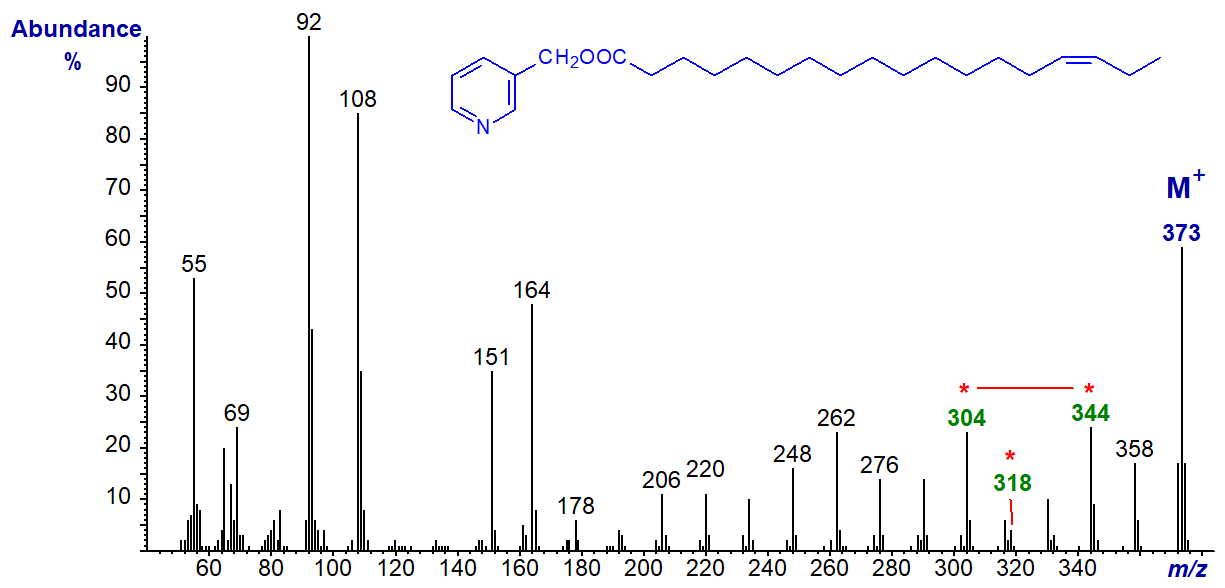
3-Pyridylcarbinyl 16-octadecenoate (16-18:1) (Christie et al., 1987). With this and the 17-isomer that follows, it is not difficult to recognize that the double bond must be close to the terminal methyl group because of the regular series of ions 14 amu apart before it, but it helps to have the model spectra available to be sure of the exact position. Location of double bonds near the terminal position is a problem with all types of nitrogen-containing derivative.
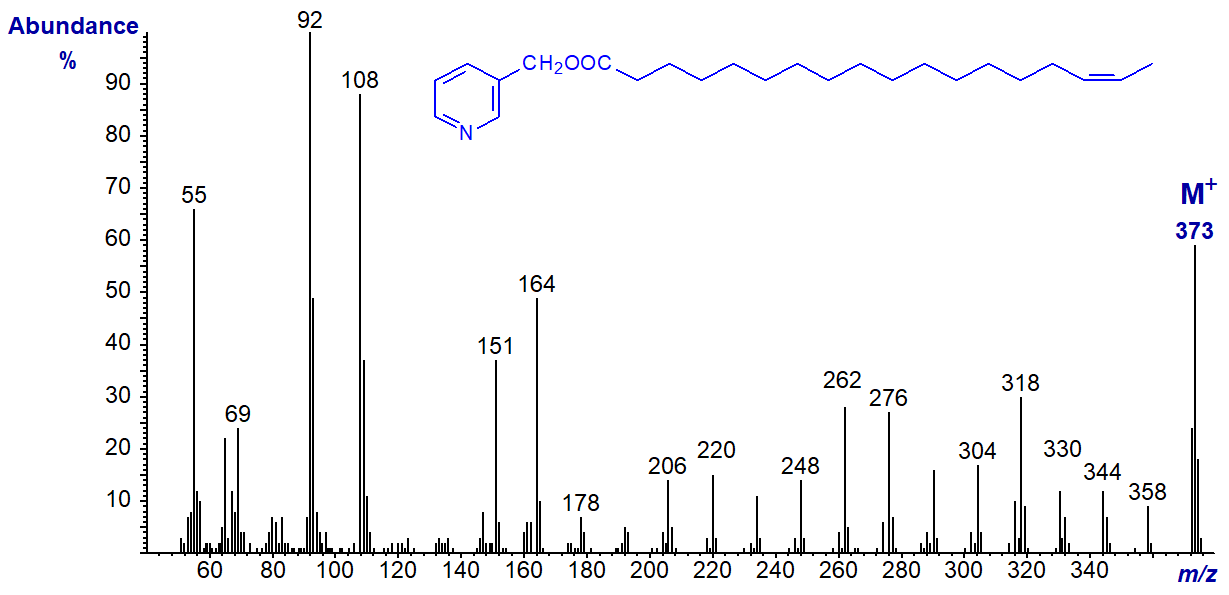
3-Pyridylcarbinyl 17-octadecenoate (17-18:1) - the gap of 41 amu between m/z = 332 and 373 at the end of the spectrum may be characteristic, but we have no other spectra of homologues with terminal double bonds for comparison.
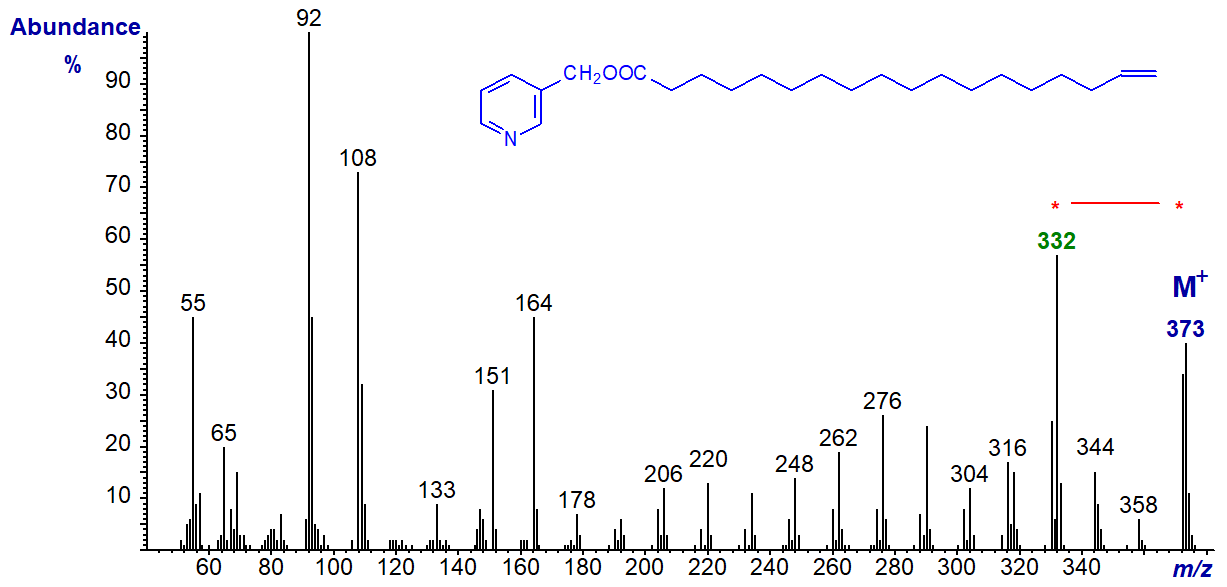
Of course, if the 17-double bond is located more centrally in the chain, there is no difficulty with interpretation as with the spectrum of 3‑pyridylcarbinyl 17‑tetracosenoate (17-24:1) -
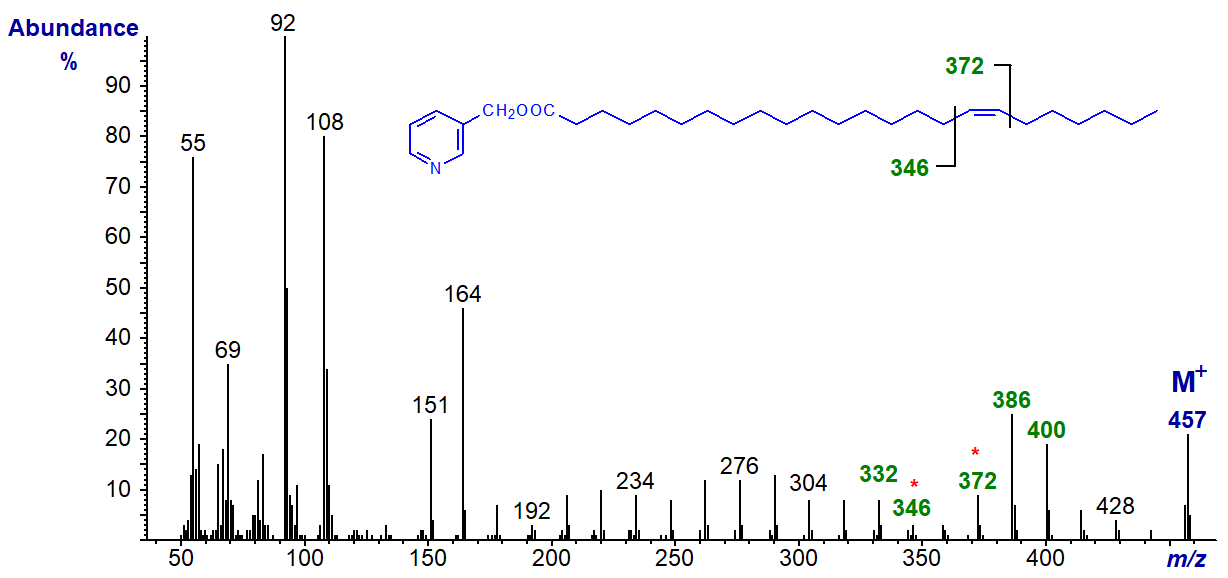
The double bond can be located by the gap of 26 amu between m/z = 346 and 372, or the gap of 40 amu between m/z = 332 and 372, with the doublet of ions at m/z = 386 and 400 providing valuable supporting evidence.
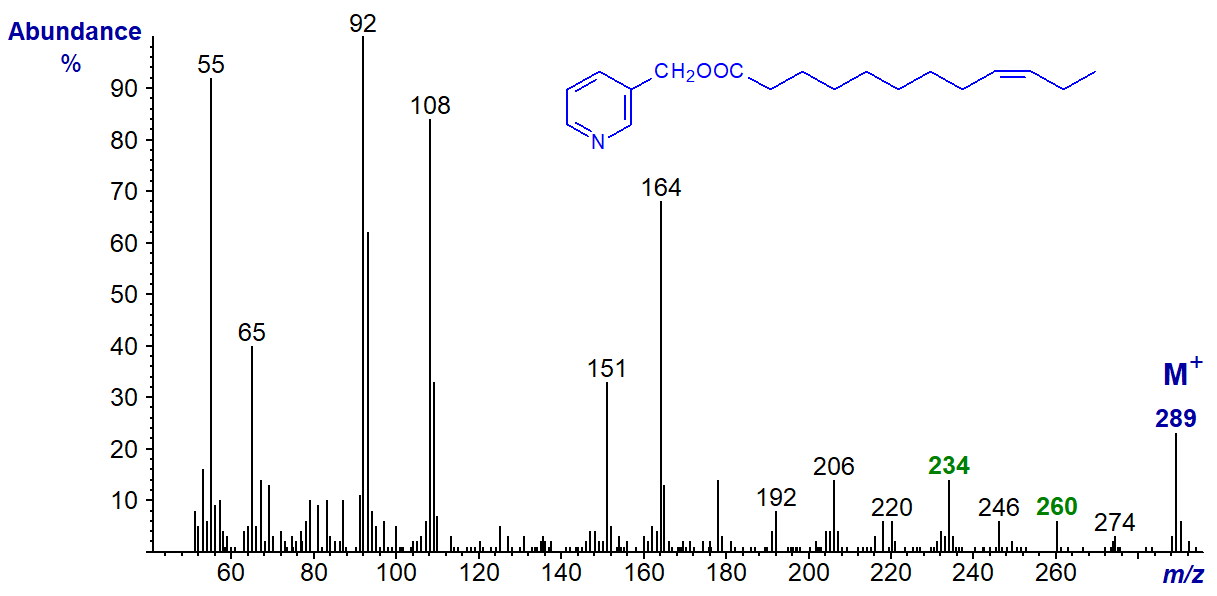
Similarly, the mass spectrum of the much shorter chain 3-pyridylcarbinyl dodec-9-enoate (9-12:1) shows that the double bond can be located here by the ions at m/z = 234 and 260 (see the spectrum of 3-pyridylcarbinyl oleate above - Figure 1).
Spectra of 3-pyridylcarbinol esters of many more monoenoic fatty acids are illustrated in our Archive section, but without interpretation. Most of these have not been formally published elsewhere.
Branched-Chain Monoenoic Fatty Acids
Monomethyl-branched-chain monoenoic fatty acids are not very common in nature, but we have mass spectra of 3-pyridylcarbinol esters of a few fatty acids of this type (iso- and anteiso-methyl) from marine sources. Interpretation is usually straight forward. Positions of double bonds are confirmed as described above and those for methyl branches by gaps of 28 amu for loss of the carbon carrying the methyl branch (see the pages on 3-pyridylcarbinol esters of saturated branched-chain fatty acids. None of these spectra have been published formally elsewhere to my knowledge.
3-Pyridylcarbinyl 14-methyl-hexadec-6-enoate (anteiso-methyl-6-16:1) -
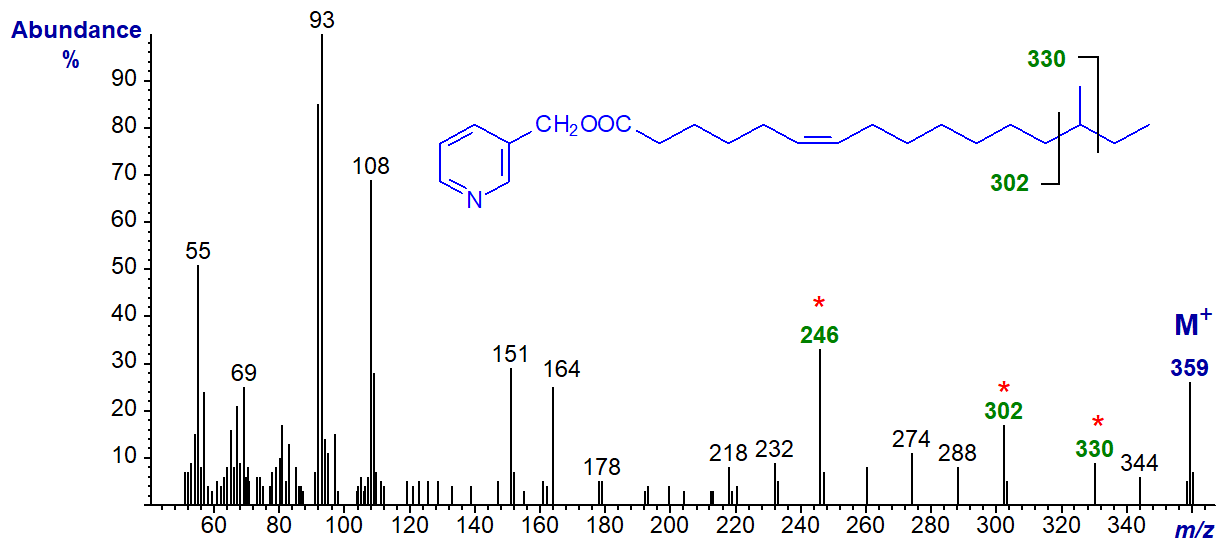
The methyl branch is located by the gap of 28 amu between m/z = 302 and 330, and the double bond in position 6 is most easily recognized by the ions around the fingerprint ion at m/z = 246 (see the spectrum of 3-pyridylcarbinyl petroselinate (6-18:1) above).
3-Pyridylcarbinyl 15-methyl-hexadec-6-enoate (iso-methyl-6-16:1) -
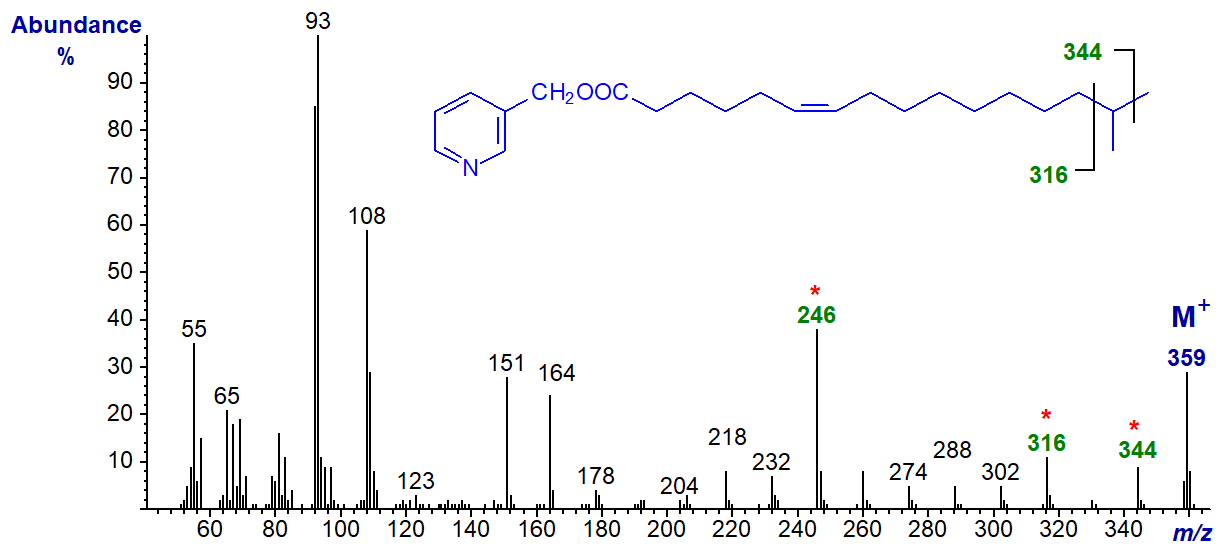
Here, the methyl branch is located by the gap shifted to between m/z = 316 and 344, but the double bond is in the same position as the previous spectrum.
3-Pyridylcarbinyl 15-methyl-hexadec-9-enoate (iso-methyl-9-16:1) -
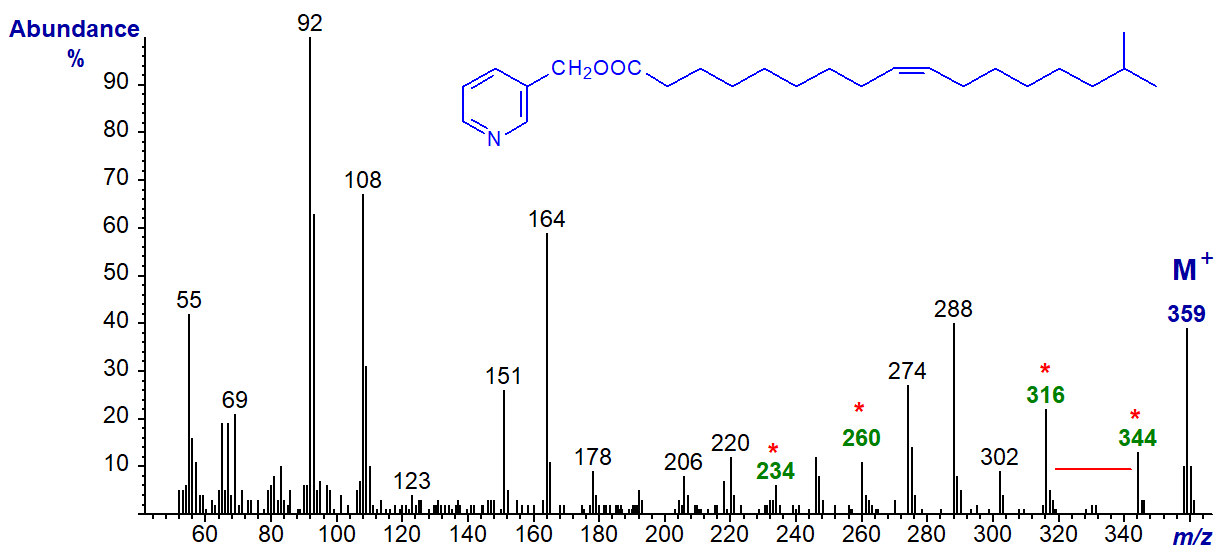
The methyl branch is located as in the last spectrum, but the double bond in recognized by the characteristic group of ions in the range m/z = 220 to 288 (see the spectrum of 3‑pyridylcarbinyl oleate above - Figure 1).
Spectra of 3-pyridylcarbinol esters of more branched-chain monoenoic fatty acids are illustrated in our Archive section, but without interpretation. Only a few of these may have been published elsewhere.
References
- Christie, W.W., Brechany, E.Y. and Holman, R.T. Mass spectra of the picolinyl esters of isomeric mono- and dienoic fatty acids. Lipids, 22, 224-228 (1987); DOI.
- Gunstone, F.D. and Ismail, I.A. Fatty acids. Part 13. The synthesis of all the cis n-octadecenoic acids. Chem. Phys. Lipids, 1, 209-224 (1967); DOI.
- Harvey, D.J. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom., 9, 33-38 (1982); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
