Mass Spectrometry of 3-Pyridylcarbinol Esters
Saturated Fatty Acids
Introduction
 Several nitrogen-containing derivatives of fatty acids
have been described with excellent mass spectrometric properties in that they enable double bonds,
branch-points, and other structural features to be located in the alkyl chain with relative ease.
Although I do not wish to be dogmatic on the subject, in my opinion, 3‑pyridylcarbinol
('picolinyl') esters are the best single derivatives of this type in mass spectrometry terms at least.
Their GC properties are far from ideal, but many of the modern polar stationary phases have sufficient thermally stability
to afford good resolution.
That said, dimethyloxazoline (DMOX) and pyrrolidide derivatives are also very useful, and they can be better
than 3‑pyridylcarbinol esters in some circumstances.
For example, DMOX derivatives have much better GC properties and are particularly suited to location of conjugated double bond systems.
Rather than considering them as competitors for the preferred derivative, I treat them as valuable alternatives that
provide useful complementary data.
Faced with a novel sample, it is always useful to prepared more than one type of derivative for analysis by GC-MS.
D.J. Harvey was responsible for much of the early work on 3‑pyridylcarbinol esters, and his review article is
still very valuable (Harvey, 1992).
Several nitrogen-containing derivatives of fatty acids
have been described with excellent mass spectrometric properties in that they enable double bonds,
branch-points, and other structural features to be located in the alkyl chain with relative ease.
Although I do not wish to be dogmatic on the subject, in my opinion, 3‑pyridylcarbinol
('picolinyl') esters are the best single derivatives of this type in mass spectrometry terms at least.
Their GC properties are far from ideal, but many of the modern polar stationary phases have sufficient thermally stability
to afford good resolution.
That said, dimethyloxazoline (DMOX) and pyrrolidide derivatives are also very useful, and they can be better
than 3‑pyridylcarbinol esters in some circumstances.
For example, DMOX derivatives have much better GC properties and are particularly suited to location of conjugated double bond systems.
Rather than considering them as competitors for the preferred derivative, I treat them as valuable alternatives that
provide useful complementary data.
Faced with a novel sample, it is always useful to prepared more than one type of derivative for analysis by GC-MS.
D.J. Harvey was responsible for much of the early work on 3‑pyridylcarbinol esters, and his review article is
still very valuable (Harvey, 1992).
 This and
the other articles in this section describe the use of 3-pyridylcarbinol esters specifically for structure
determination of fatty acids.
In addition to analysis by GC, they can be separated by silver-ion or reversed-phase
HPLC, provided that a base-deactivated stationary phase is employed or if a small amount of pyridine is added to the mobile phase with
the latter (Christie and Stefanov, 1987; Christie, 1998), and these are useful techniques to enrich minor
components (see our web page on Concentration of minor components).
Methods for preparing the derivatives are described elsewhere in our website in a separate
document on this site.
Although I have no personal experience of the technique, 3-pyridylcarbinol esters have been used to determine double bond positions and
configuration in unsaturated fatty acids by means of gas-phase infrared spectroscopy (Kirschbaum, C. et al., 2023)
This and
the other articles in this section describe the use of 3-pyridylcarbinol esters specifically for structure
determination of fatty acids.
In addition to analysis by GC, they can be separated by silver-ion or reversed-phase
HPLC, provided that a base-deactivated stationary phase is employed or if a small amount of pyridine is added to the mobile phase with
the latter (Christie and Stefanov, 1987; Christie, 1998), and these are useful techniques to enrich minor
components (see our web page on Concentration of minor components).
Methods for preparing the derivatives are described elsewhere in our website in a separate
document on this site.
Although I have no personal experience of the technique, 3-pyridylcarbinol esters have been used to determine double bond positions and
configuration in unsaturated fatty acids by means of gas-phase infrared spectroscopy (Kirschbaum, C. et al., 2023)
Nomenclature: It should be noted that for most of the 40 years or so since they were first described, 3-pyridylcarbinol esters have been incorrectly termed ‘picolinyl’ esters in the scientific literature, not least by myself regretfully (picolinyl alcohol is in fact 2-pyridylcarbinol not the 3-isomer). The correct trivial name is ‘nicotinyl’ esters, but all dubiety is removed if they are given the more systematic name ‘3-pyridylcarbinol’ or (‘3‑pyridinylmethyl’) esters.
Some Mechanistic Considerations
The mass spectrum of 3-pyridylcarbinyl palmitate (hexadecanoate or 16:0) (Harvey, 1982) is illustrated -
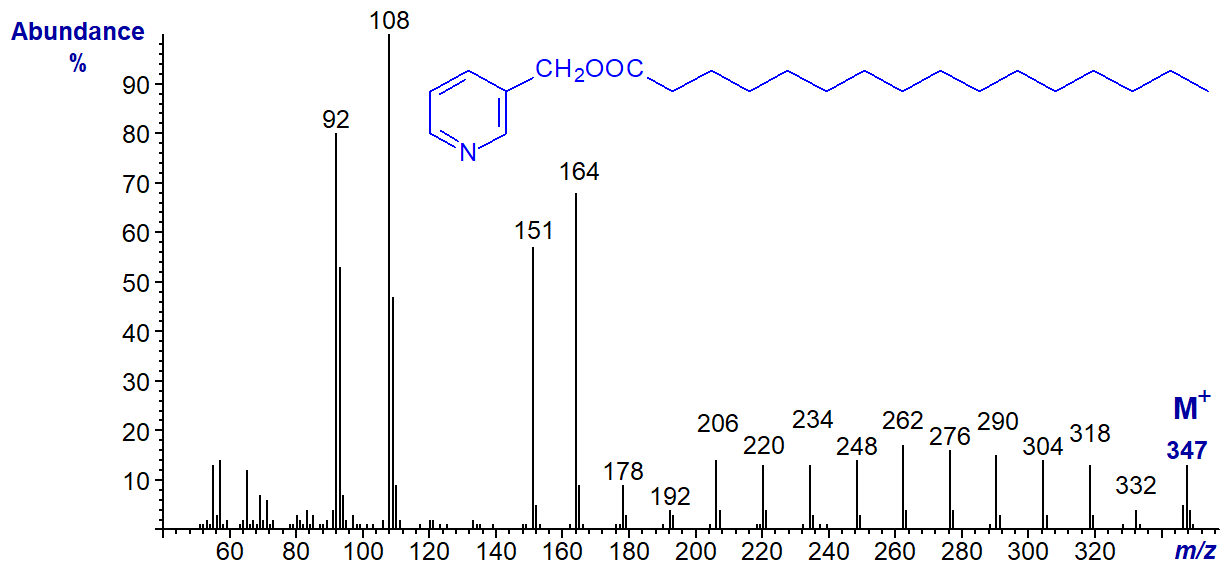
It is typical in that it has prominent ions at m/z = 92, 108, 151 and 164, which are all fragments about the pyridine ring (if any of these ions is missing from a spectrum it may be indicative of a functional group adjacent to the carboxyl moiety). The molecular ion (m/z = 347) is easily distinguished, and it is always odd numbered because of the presence of the nitrogen atom, but most other ions are even numbered. Ions below m/z = 92 can usually be ignored.
In interpreting such spectra, the simplest approach is to start with the molecular ion and progress downward, as if one were unzipping the molecule one methylene group at a time. Thus, there is loss of a methyl group to m/z = 332, followed by a series of ions 14 amu apart for loss of successive methylene groups, i.e., m/z = 318, 304, 290, 276 and so forth. There is little sign of the complex rearrangement ions that can be found with such fatty acid derivatives when subjected to mass spectrometry in the form of methyl esters.
The main fragmentations are often portrayed simplistically as cleavages at the points shown, but in reality, studies with fatty acid derivatives labelled with stable isotopes have shown that those ions close to the carboxyl group can involve some rearrangement with specific hydrogen abstractions (Harvey, 1992; Hamilton and Christie, 2000; Yang et al., 2006).
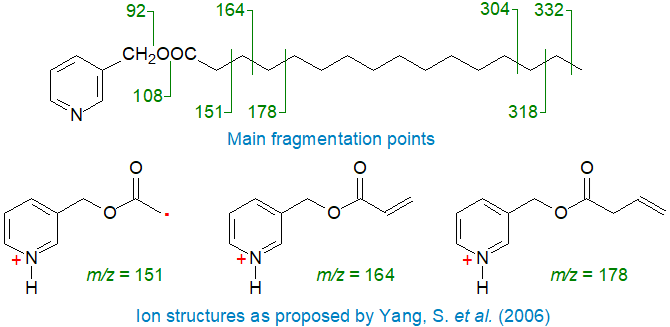 |
| Mass spectral fragmentations for 3-pyridylcarbinyl palmitate. |
In particular, the ion at m/z = 151 is not formed simply by the well-known McLafferty rearrangement as is usually proposed (see our web pages on Methyl esters of saturated fatty acids for a discussion of the McLafferty mechanism). Rather, Yang and colleagues (2006) suggest transfer of a hydrogen ion from carbon 3 to the nitrogen atom prior to cleavage to give the ion illustrated. For example, the McLafferty rearrangement would require a transfer of a hydrogen atom from carbon 4 to a cyclic intermediate, but the spectrum of the 4,4‑dideutero-palmitate derivative shows that this cannot be so; otherwise, the ion at m/z = 151 would be shifted to m/z = 152.
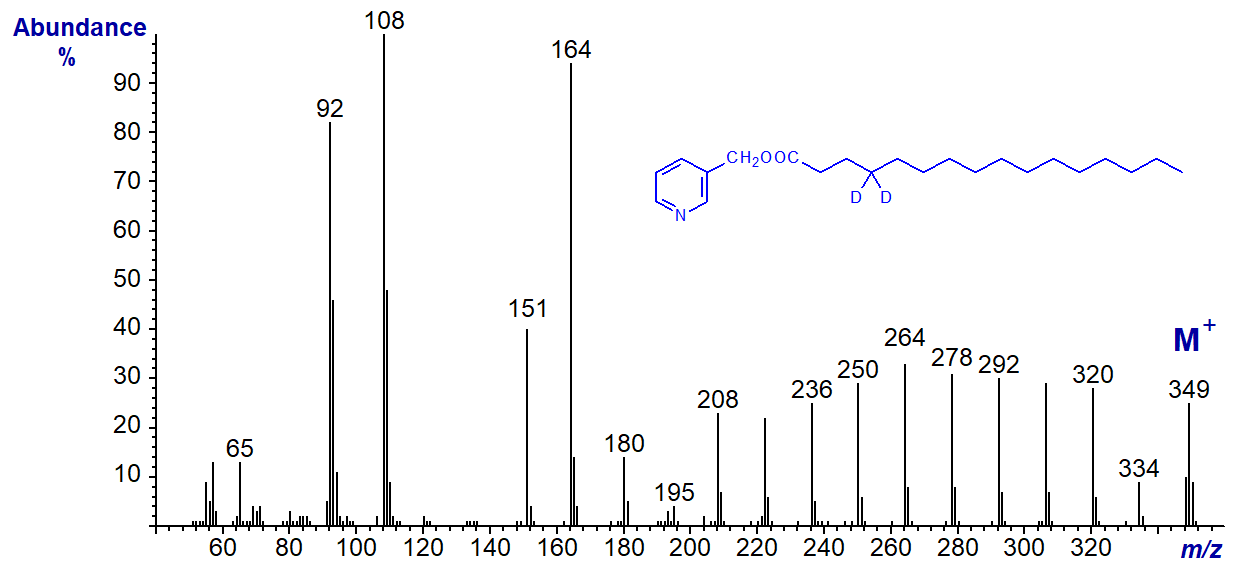
Similarly, formation of the ions at m/z = 164 and 178 require distinctive and unexpected rearrangements (Hamilton and Christie, 2000; Yang et al., 2006), but those ions further down the saturated chain probably represent simple radical-induced cleavage. As these web pages are not intended as detailed mechanistic accounts, fragmentations are generally represented in a simplified manner here. Those with an interest in fragmentation mechanisms will find that a study of the spectra of the saturated deuterated derivatives in our Archive page, especially of 2,2-D-16:0 and 3,3D-16:0, together with the publications cited here will be a useful exercise.
Further Representative Mass Spectra
There follow spectra for 3-pyridylcarbinyl decanoate (10:0) -
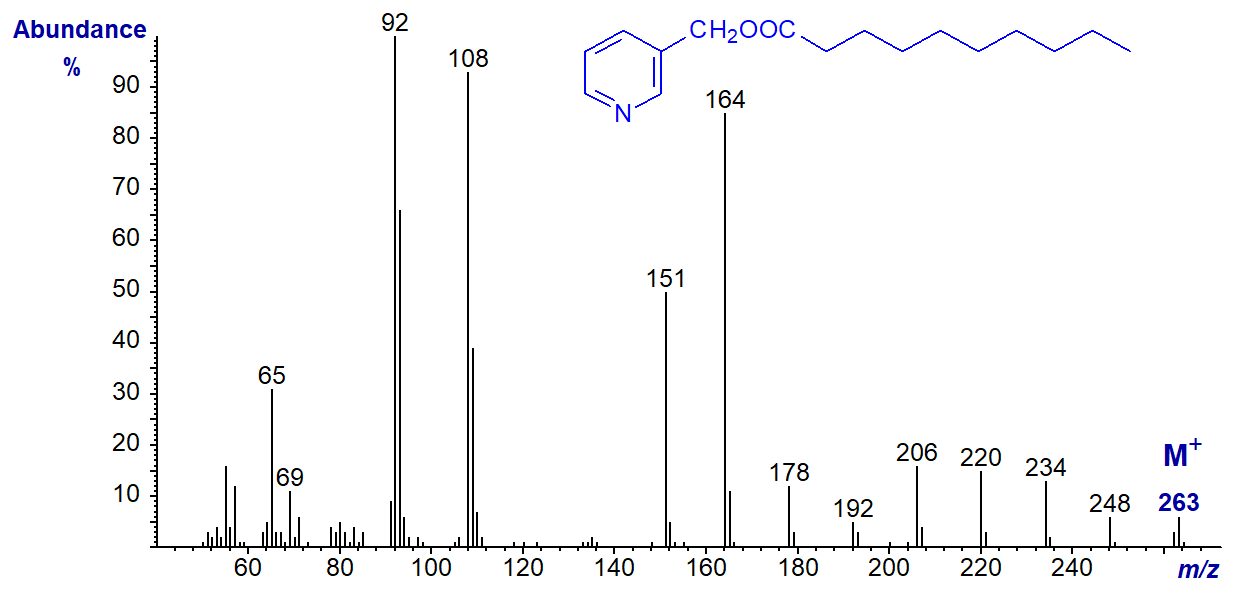
- and 3-pyridylcarbinyl tetracosanoate (24:0), which show essentially the same features, i.e., the regular series of ions 14 amu apart -
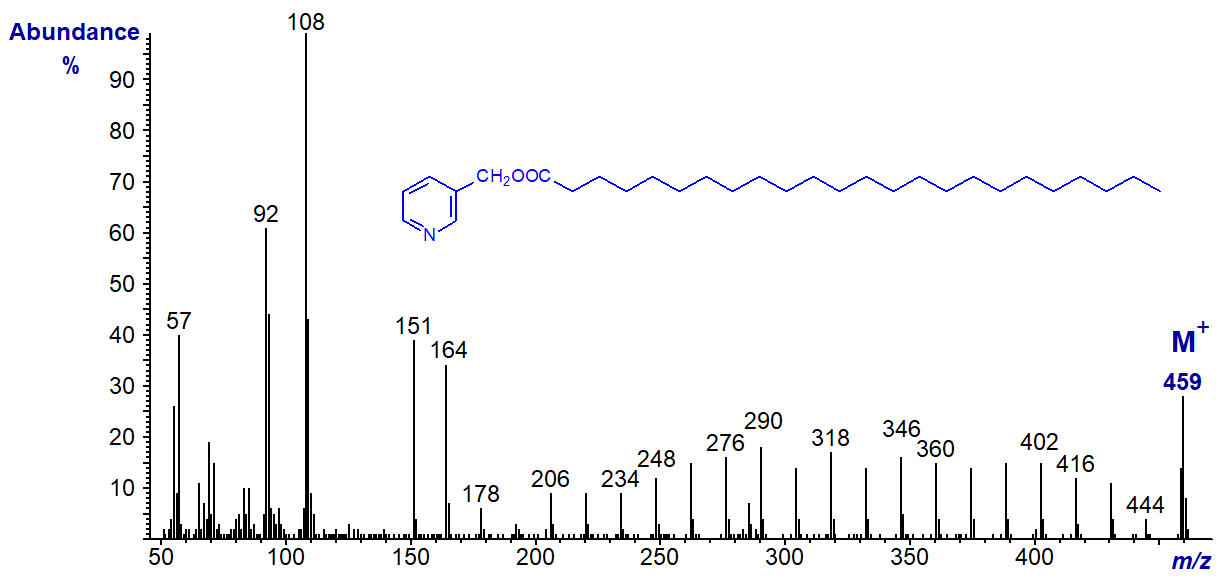
Then the spectrum of 3-pyridylcarbinyl butyrate (4:0) -
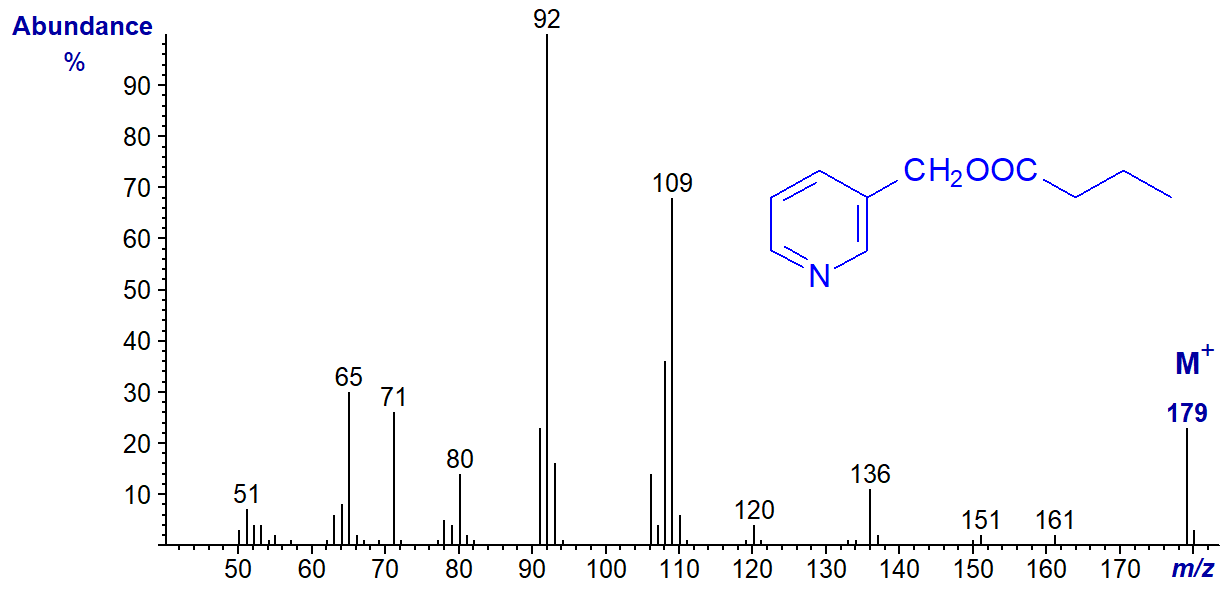
In this instance, the expected ions at m/z = 151 and 164 are essentially absent, as the requisite cyclic intermediates cannot be formed.
We have spectra on file for 3-pyridylcarbinol esters of straight-chain fatty acids from 2:0 to 30:0, including nearly all the odd-chain ones and others labelled with stable isotopes. These can be accessed (but without interpretation) from our Archive page. Only a few of them have been published formally elsewhere.
References
- Christie, W.W. Structural analysis of fatty acids. In: Advances in Lipid Methodology - Four, pp. 119-169 (edited by W.W. Christie, Oily Press, Dundee) (1997).
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998); DOI.
- Christie, W.W. and Stefanov, K. Separation of picolinyl ester derivatives of fatty acids by high-performance liquid chromatography for identification by mass spectrometry. J. Chromatogr. A, 392, 259-265 (1987); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Harvey, D.J. Mass spectrometry of picolinyl and other nitrogen-containing derivatives of lipids. In: Advances in Lipid Methodology - One, pp. 19-80 (edited by W.W. Christie, Oily Press, Ayr) (1992).
- Kirschbaum, C. and others. Establishing carbon-carbon double bond position and configuration in unsaturated fatty acids by gas-phase infrared spectroscopy. Chem. Sci., 14, 2518-2527 (2023); DOI.
- Yang, S., Minkler, P., Hoppel, C. and Tserng, K.-Y. Picolinyl ester fragmentation mechanism studies with application to the identification of acylcarnitine acyl groups following transesterification. J. Am. Soc. Mass Spectrom., 17, 1620-1628 (2006); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
