Mass Spectrometry of 3-Pyridylcarbinol Esters
Dienoic Fatty Acids. Part 2. Conjugated and
Bis- and Polymethylene-Interrupted Dienes
In this web page, the electron-impact mass spectra of 3-pyridylcarbinol ('picolinyl') esters of those dienoic fatty acids with conjugated double bond systems or with two or more methylene groups between the double bonds are described. Dienes Part 1 describes the spectra of the 3‑pyridylcarbinol esters of the more common dienoic acids with methylene-interrupted double bonds. The corresponding web page on monoenes provides an explanation of the principles involved (we are normally looking for gaps of 26 or 40 amu to locate double bonds), while that on saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.).
There is a separate document dealing with mass spectra of 3-pyridylcarbinol esters of allenic fatty acids, which can be considered to be dienoic. As with other web pages on this site, fragmentations are illustrated over simplistically as this is intended as a practical as opposed to a mechanistic guide.
Conjugated Dienoic Fatty Acids
3-Pyridylcarbinol esters may not be the best derivatives for structural analysis of fatty acids with conjugated double bond systems, and DMOX derivatives may be better in this instance (see DMOX derivatives - dienoic fatty acids). MTAD adducts of methyl esters (Diels-Alder reaction) are uniquely valuable, especially for the complex mixtures found in commercial ‘conjugated linoleic acid’ (CLA) and some natural samples, and they afford a different approach to analysis. However, 3-pyridylcarbinol esters are still useful if authentic standards are available for comparison, especially when isomers are well resolved on the GC column.
The mass spectrum of 3-pyridylcarbinyl 9-cis,11-trans-octadecadienoate, the most abundant natural isomer (e.g., in cows' milk fat) follows -
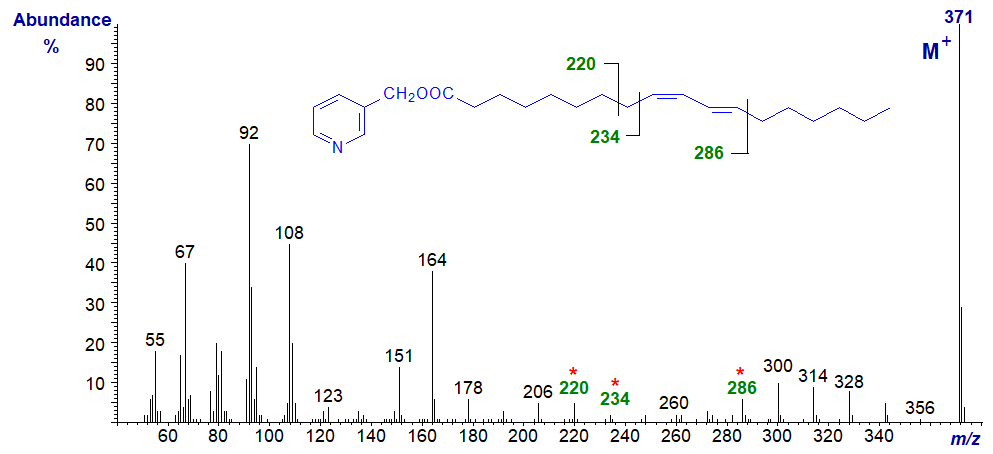
There is a gap of 52 amu between m/z = 234 and 286 at either end of the conjugated double bond system, with intervals of 14 amu on either side for cleavage at successive methylene groups. The molecular ion at m/z = 371 is the base ion. Of course, there is no information on the geometry of the double bonds.
3-Pyridylcarbinyl 10-trans,12-cis-octadecadienoate (from a commercial CLA preparation) -
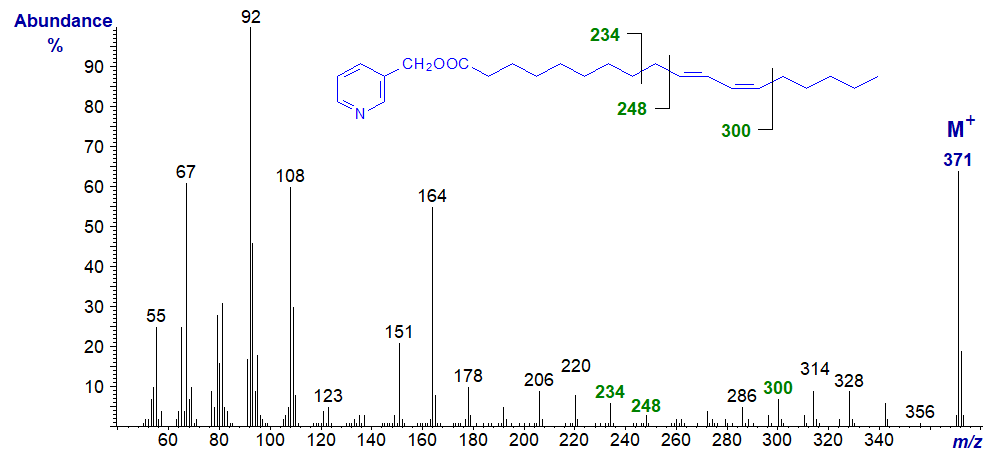
In this instance, the diagnostic ions are shifted up by 14 amu from the previous spectrum, but not very convincingly if a definitive identification were to be required in the absence of a standard spectrum. These spectra do not appear to have been published formally elsewhere.
Bis-Methylene-Interrupted Dienoic Fatty Acids
It is becoming apparent that bis- and polymethylene-interrupted dienoic fatty acids are more common in nature than may have been supposed. Fatty acids with a 5,9‑double bond system and their chain elongation products are common in seed oils from Gymnosperms and in many marine invertebrates such as sponges. The spectrum of 5,9-18:2 and of some synthetic fatty acids with the bis-methylene-interrupted double bonds (useful for confirming fragmentation mechanisms) are illustrated next (Christie et al., 1987).
3-Pyridylcarbinyl 5,9-octadecadienoate (5,9-18:2) (Hierro et al., 1996) -
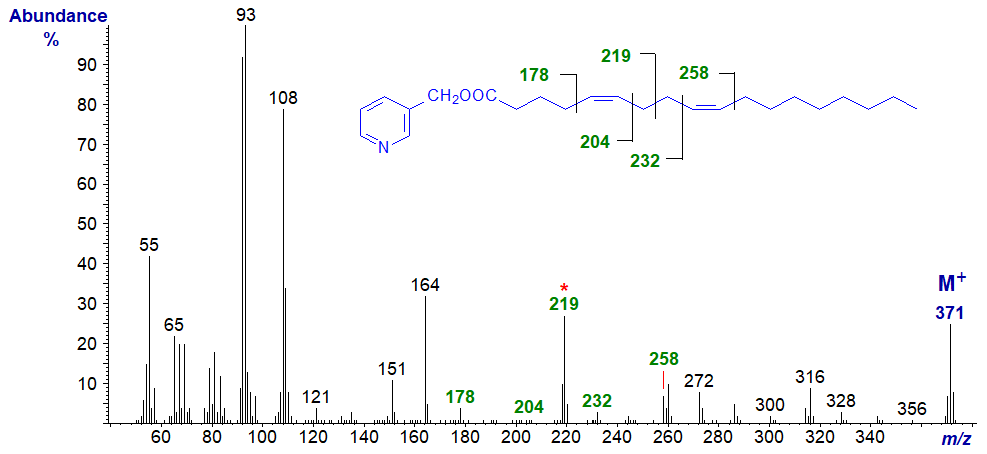
The truly distinctive feature is the ion at m/z = 219 (unusual for a pyridylcarbinol ester in being odd-numbered) representing cleavage at the centre of the bis-methylene-interrupted double bond system. In addition, gaps of 26 amu, between m/z = 178 and 204 and m/z = 232 and 258 help to locate the double bonds in positions 5 and 9, respectively, although the gaps of ~40 amu (m/z = 178 to 219 to 258) are perhaps more convincing.
3-Pyridylcarbinyl 2,6-octadecadienoate (2,6-18:2) -
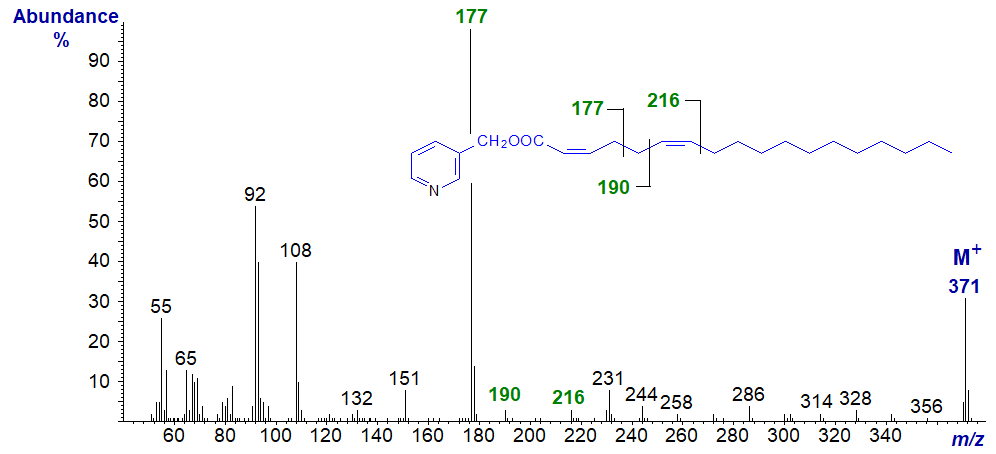
In this spectrum (unpublished), the ion at m/z = 177 representing cleavage at the centre of the bis‑methylene-interrupted double bonds is the base ion in fact. The expected ion at m/z = 164 is missing as with other fatty acids with a double bond in position 2, and the ions at m/z = 190 and 216 confirm the position of the double bond in position 6.
3-Pyridylcarbinyl 6,10-octadecadienoate (6,10-18:2) (Christie et al., 1987) -
All the ions that locate the double bonds (marked) are easily located, and the same is true for the 7,11-isomer in our Archive. It appears that ions diagnostic for the positions of the double bonds become easier to locate as they move further from the carboxyl group.
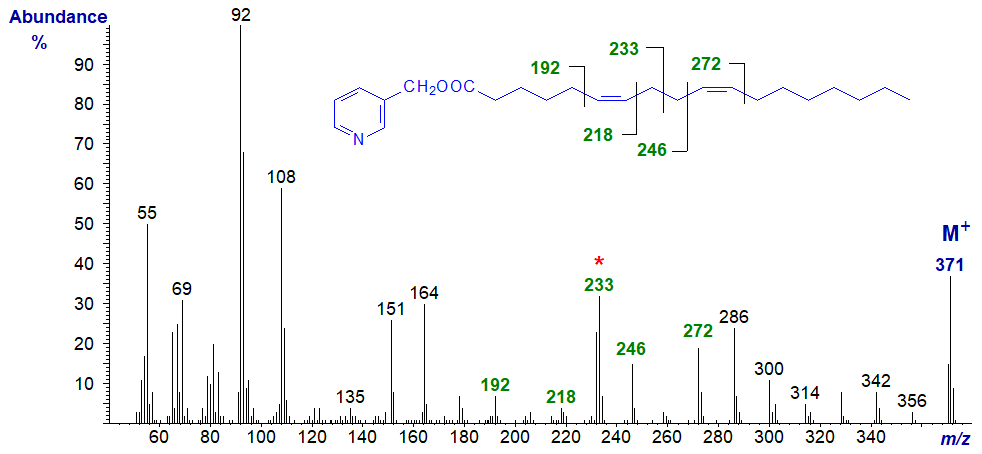
3-Pyridylcarbinyl 8,12-octadecadienoate (8,12-18:2) (Christie et al., 1987) -
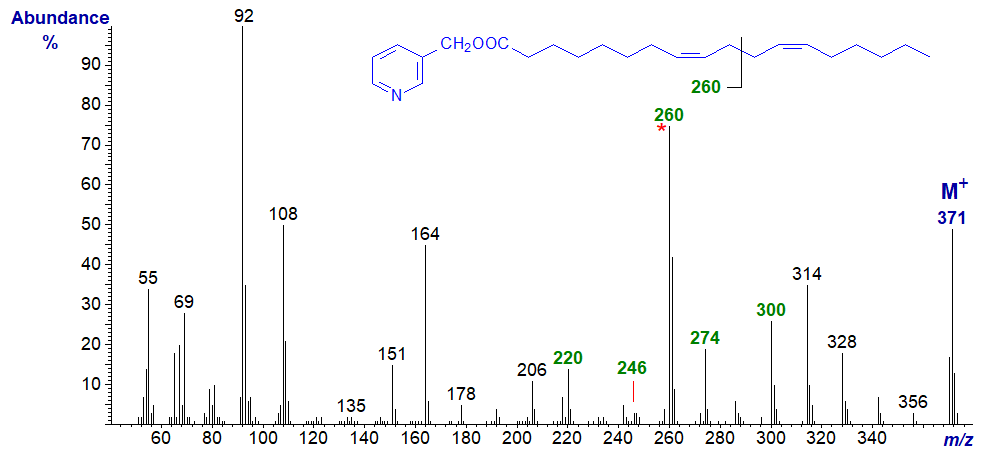
Note that the ion representing cleavage at the centre of the bis-methylene-interrupted double bonds is now even numbered (m/z = 260). Comparable features are seen in the spectrum of the 9,13-isomer as in 3-pyridylcarbinyl 9,13-eicosadienoate (9,13-20:2) (here..) and when the double bonds are even more remote from the carboxyl group as in the spectrum of 3-pyridylcarbinyl 17,21-octacosadienoate (17,21-28:2) - illustrated next.
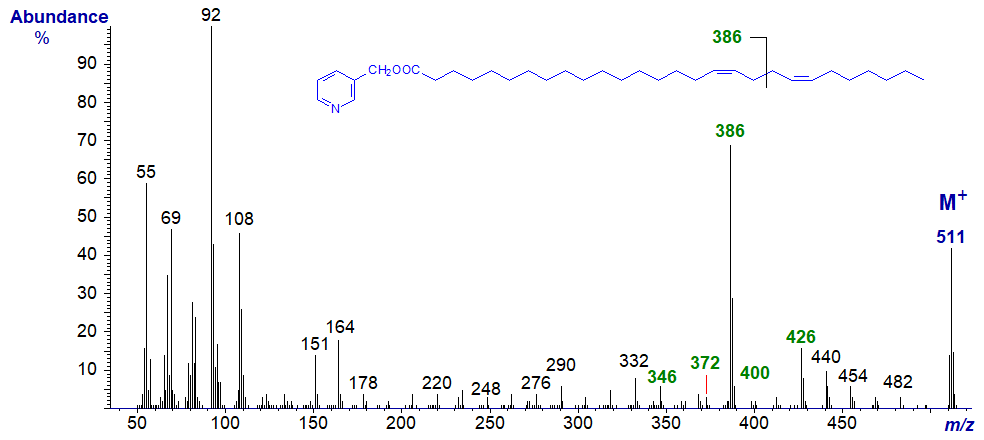
And with the spectrum of 3-pyridylcarbinyl 22-methyl-octacosa-5,9-dienoate -
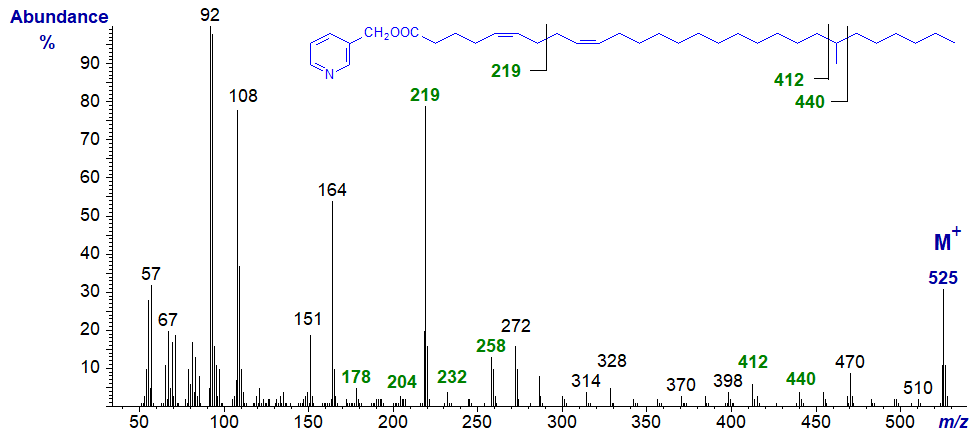
The expected ions for the 5,9-double bond system are present, and now there is an additional feature - a gap of 28 amu between m/z = 412 and 440 for the methyl branch in position 22 (see our web page on 3-pyridylcarbinol esters of branched derivatives).
Spectra of 3-pyridylcarbinol esters of many more bis-methylene-interrupted dienoic fatty acids are illustrated in our Archive section, but without interpretation. Most of these have not been formally published elsewhere.
Poly-Methylene-Interrupted Dienoic Acids
Fatty acids with more than two methylene groups between double bonds are perhaps less common than bis-methylene-interrupted isomers in nature, but some representative mass spectra of 3-pyridylcarbinol esters of synthetic and natural fatty acids of this type are illustrated below. Spectra from two synthetic fatty acids are available (Christie et al., 1987), and the first of these has been found naturally in a sponge (Carballeira and Maldona, 1989).
3-Pyridylcarbinyl 6,11-octadecadienoate (6,11-18:2) has three methylene groups between the double bonds -
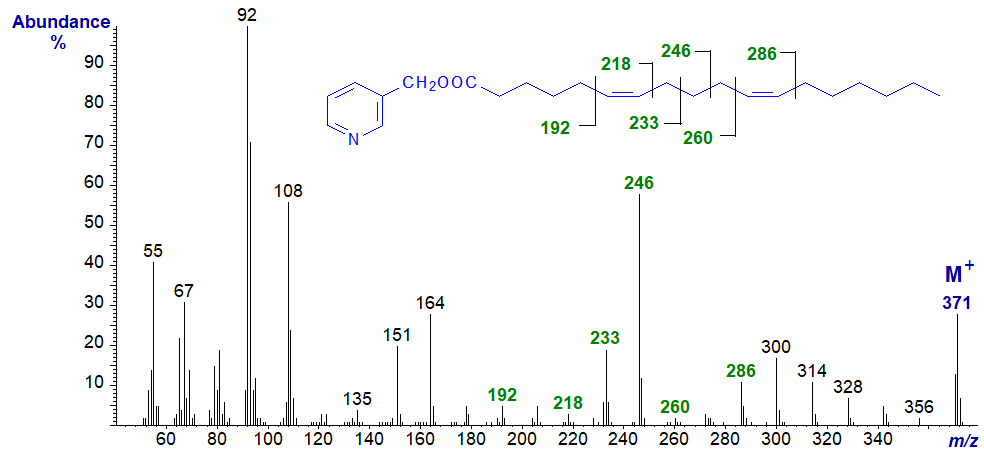
There are prominent ions for cleavages between the double bonds, i.e., at m/z = 233 and 246 (first odd-numbered), and the double bonds themselves can be located by the usual means as marked.
With 3-Pyridylcarbinyl 7,12-octadecadienoate (7,12-18:2) -
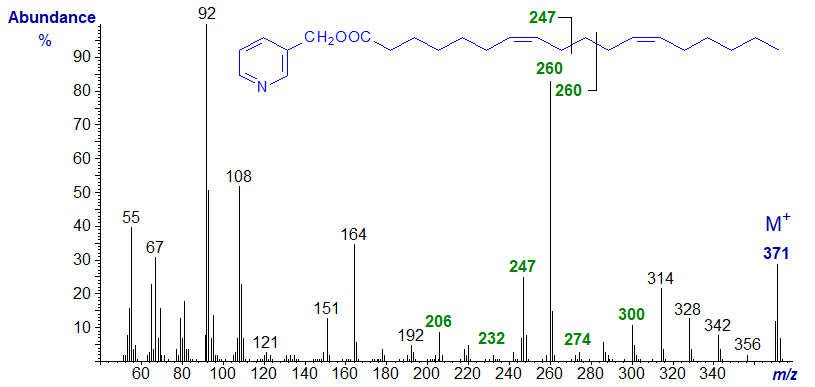
The spectrum is very similar to the previous, except that the key ions are all shifted by 14 amu upwards, as might be expected.
3-Pyridylcarbinyl 5,11-octadecadienoate (5,11-18:2) has four methylene groups between the double bonds and is present in the seeds of Gingko biloba. Its mass spectrum has been published (Wolff et al., 1999) -
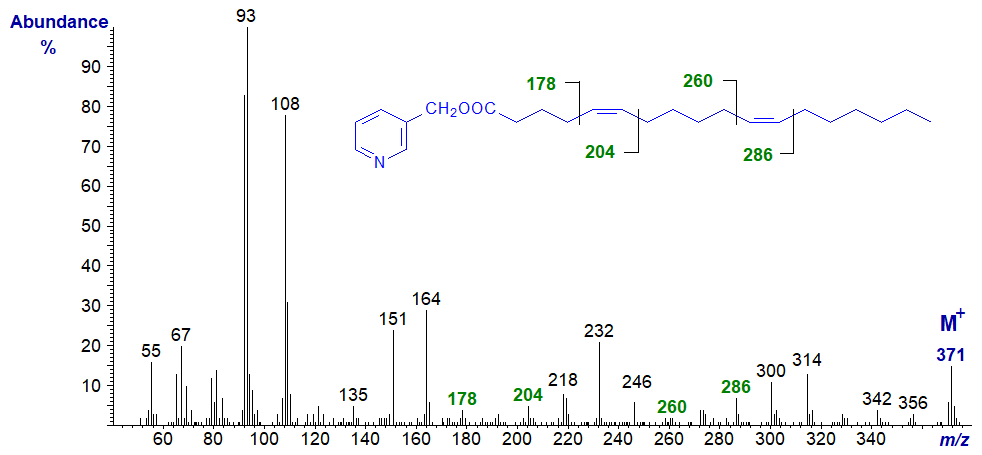
In this instance, the double bonds are located in the same way as for isolated double bonds as in monoenoic fatty acid derivatives, with that for position 5 being determined by the gap of 26 amu between m/z = 178 and 204, and for position 11 by that between m/z = 260 and 286. Direct comparison with the mass spectrum of 5-18:1 is invaluable here, for confirmation of the position of the first double bond. It can also be helpful to look for the gap of 40 amu for the double bond and the adjacent methylene group on the carboxyl side. 3‑Pyridylcarbinyl 5,11-eicosadienoate in our Archive pages has a very similar spectrum.
With 3-Pyridylcarbinyl 6,12-octadecadienoate (6,12-18:2) -
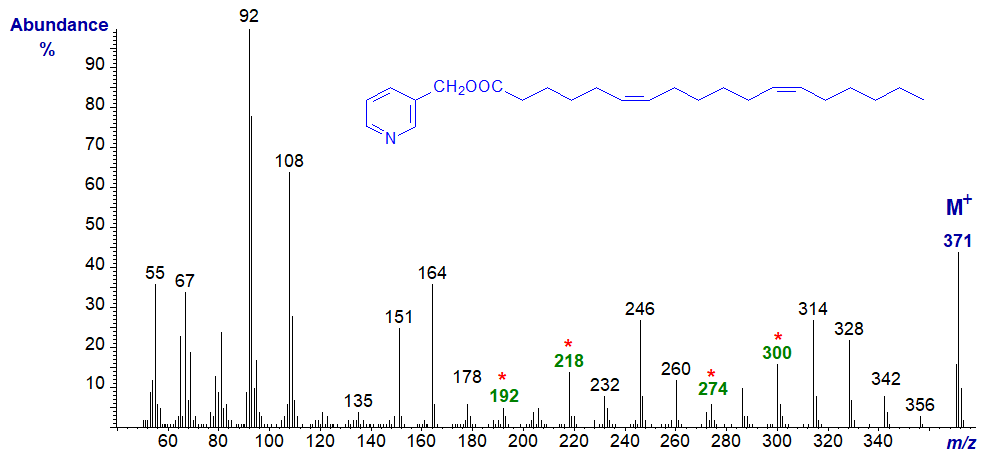
This spectrum resembles that of the 5,11-isomer except that the diagnostic ions are all 14 amu higher.
My colleagues and I have encountered a few long-chain fatty acids with more than four methylene groups between double bonds and some representative spectra from marine invertebrates follow.
3-Pyridylcarbinyl 5,13-eicosadienoate (5,13-20:2) (Christie et al., 1988). As with the previous, interpretation of the spectrum is like that for isolated double bonds as in monoenes, and this is also true of the spectrum that follows.
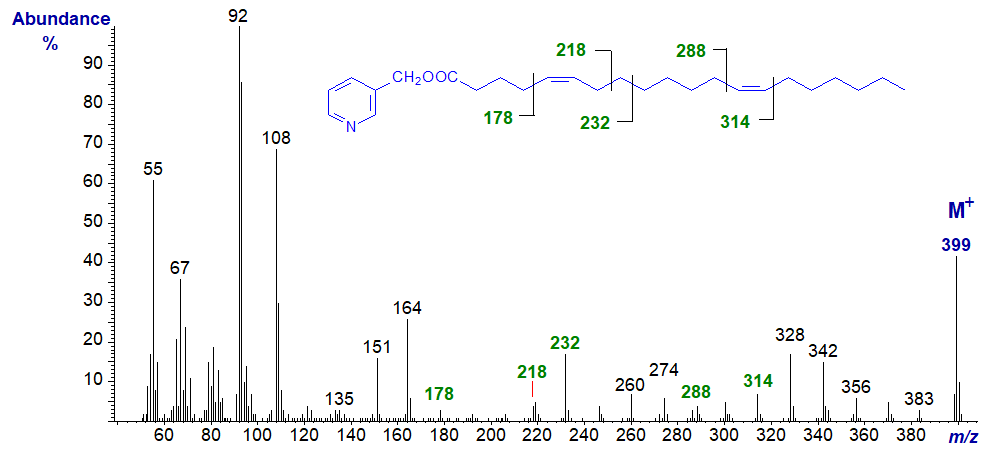
3-Pyridylcarbinyl 9,19-hexacosadienoate (9,19-26:2) (Christie et al., 1992) -
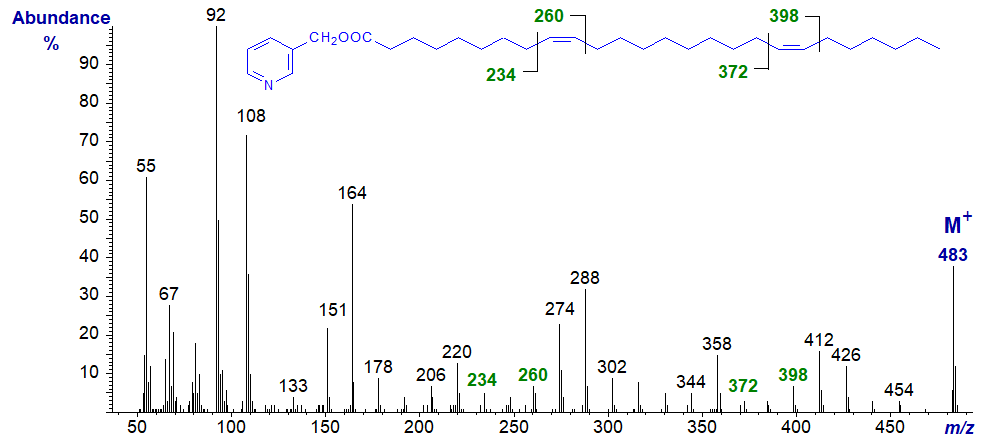
Spectra of 3-pyridylcarbinol esters of many more dienoic fatty acids, including some branched-chain isomers, are illustrated in our Archive section, but without interpretation. Most of these have not been published formally elsewhere.
References
- Carballeira, N.M. and Maldona, M.E. On the isolation of the new fatty acid 6,11-eicosadienoic (20:2) and related 6,11-dienoic acids from the sponge Euryspongia rosea. Lipids, 24, 665-668 (1989); DOI.
- Christie, W.W., Brechany, E.Y., Gunstone, F.D., Lie Ken Jie, M.S.F. and Holman, R.T. Mass spectra of the picolinyl esters of some non-methylene-interrupted octadecadienoic acids. Lipids, 22, 664-666 (1987); DOI.
- Christie, W.W., Brechany, E.Y. and Stefanov, K. Silver ion high-performance liquid chromatography and gas chromatography-mass spectrometry in the analysis of complex fatty acid mixtures: application to marine invertebrates. Chem. Phys. Lipids, 46, 127-136 (1988); DOI.
- Christie, W.W., Brechany, E.Y., Stefanov, K. and Popov, S. The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids, 27, 640-644 (1992); DOI.
- Hierro, M.T.G., Robertson, G., Christie, W.W. and Joh, Y.-G. The fatty acid composition of the seeds of Ginkgo biloba. J. Am. Oil Chem. Soc., 73, 575-579 (1996); DOI.
- Wolff, R.L., Christie, W.W. and Marpeau, A.M. Reinvestigation of the polymethylene-interrupted 18:2 and 20:2 acids of Ginkgo biloba seed lipids. J. Am. Oil Chem. Soc., 76, 273-276 (1999); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
