Mass Spectrometry of 3-Pyridylcarbinol Esters
Dienoic Fatty Acids. Part 1. Methylene-Interrupted Dienes
In this and the next web page, the electron-impact mass spectra of 3-pyridylcarbinol esters of those dienoic fatty acids that are most likely to be encountered in nature are described, together with some useful model compounds, i.e. here in Part 1 methylene-interrupted dienes. The corresponding web page on monoenes provides an explanation of the principles involved in the location of double bonds, while that on saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.).
Part 2 discusses the spectra of the 3‑pyridylcarbinol esters of most other dienoic acids, i.e., those with conjugated double bond systems and others with two or more methylene groups between the double bonds. There is a separate document dealing with mass spectra 3-pyridylcarbinol esters of allenic fatty acids, which can be considered to be dienoic. In the spectra that follow, the main points of fragmentation are illustrated simplistically as this is intended to be a practical rather than a mechanistic treatise.
Methylene-Interrupted Octadecadienoates
The mass spectra of 3-pyridylcarbinol esters of dienoic fatty acids, like those of monoenes, tend to be distinctive and permit location of the double bonds. We are looking for gaps of 26 or 40 amu. As an example, the mass spectrum of 3-pyridylcarbinyl 9,12-octadecadienoate (linoleate or 18:2(n‑6)), the most important dienoic fatty acid in nature, is illustrated first (Harvey, 1982) -
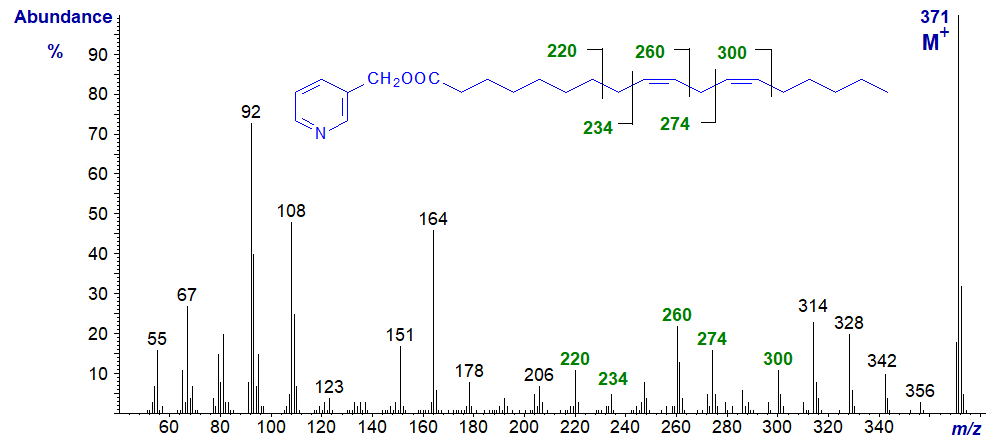
As with 3-pyridylcarbinol esters of saturated and monoenoic fatty acids, interpretation is easiest if we start with the molecular ion and work down the chain. Ions corresponding to cleavage on either side of the terminal double bond are seen at m/z = 300 and 274 (26 amu apart), there is then a gap of 14 amu to m/z = 260 and then one of 26 amu for the internal double bond to m/z = 234. When examining the spectra of unknowns, it is often easier to locate gaps of 40 amu for the double bond and the associated methylene group on the carboxyl side, i.e., in this instance between m/z = 300 and 260 and between 260 and 220. Regular series of ions 14 amu apart on either side of the double bond confirm that there are no further functional groups in these regions of the molecule.
Comparable series of ions are seen in spectra from 3-pyridylcarbinol esters of most methylene-interrupted dienes, but it is advantageous to have access to spectra of authentic standards when the double bonds are close to either end of the molecule as confusion is then most likely to arise. Details of the spectra of the complete series of isomeric methylene-interrupted octadecadienoates have been published (Christie, Brechany and Holman, 1987), but only a few of the spectra were depicted in the paper for practical reasons. All are now illustrated below, but please consult the original reference for tabulated data. These were prepared by chemical synthesis (Christie and Holman, 1967) and some may not occur in nature, although a surprising number do. Spectra of other homologous fatty acid isomers displaying the same diagnostic features may have been described elsewhere in some instances. Spectra for the complete C18 series follow.
3-Pyridylcarbinyl 2,5-octadecadienoate (2,5-18:2) (Christie et al., 1987) -
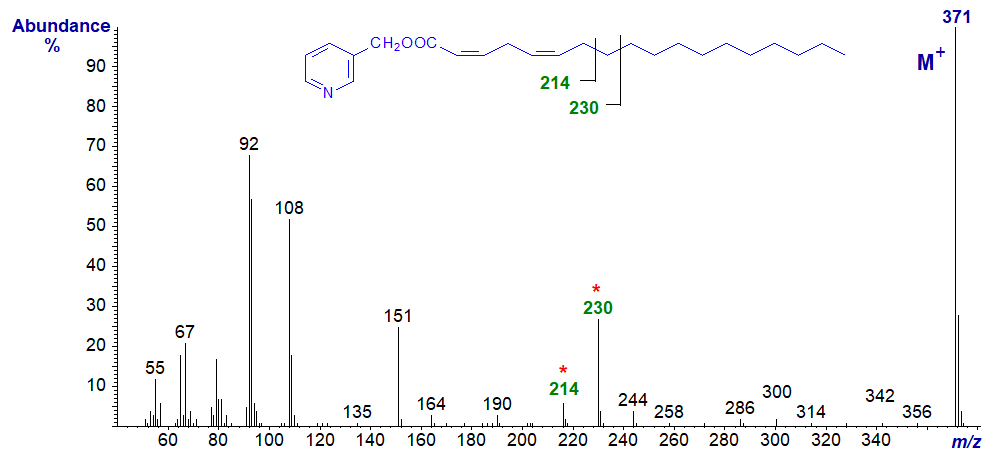
The molecular ion is the base ion, presumably because of the double bond in conjugation with the carboxyl group. Ions that identify the position of the double bonds are not apparent, but the doublet of ions at m/z = 214 and 230 is a useful indicator (see the web pages on 3‑pyridylcarbinol esters of monoenes). The regular series of ions 14 amu apart between m/z = 214 and the molecular ion confirm that there are no functional groups in this part of the alkyl chain.
3-Pyridylcarbinyl 3,6-octadecadienoate (3,6-18:2) -
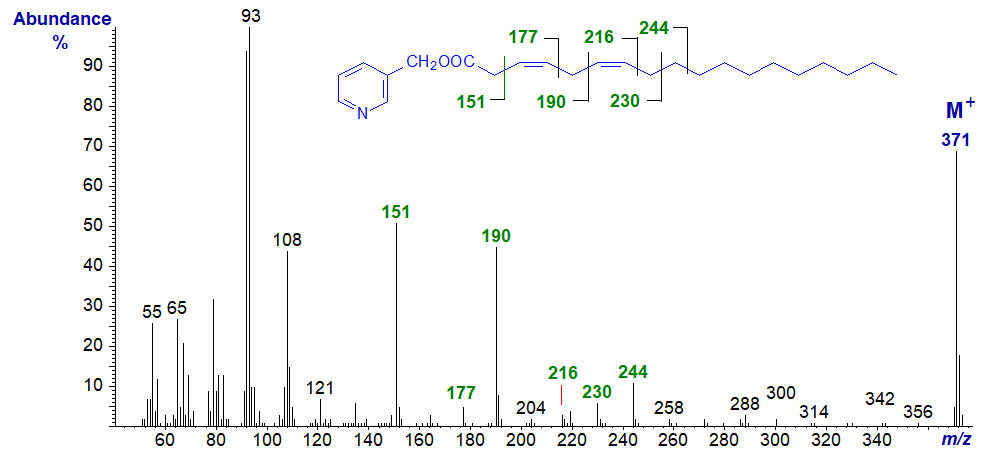
In this spectrum, the gaps of 26 amu between m/z = 151 and 177 and between 190 and 216 serve to locate the double bonds in positions 3 and 6, respectively, with the ions at m/z = 204 and 244 as further useful guides.
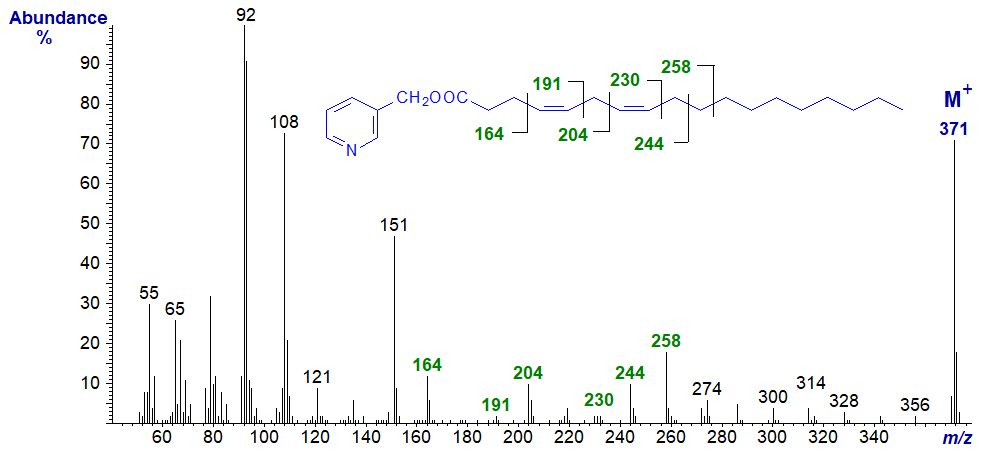
3-Pyridylcarbinyl 4,7-octadecadienoate (4,7-18:2). Some of the marked ions that locate the double bonds would be difficult to identify from first principles, so a model spectrum is essential.
3-Pyridylcarbinyl 5,8-octadecadienoate (5,8-18:2) – this fatty acid ('sebaleic') is a major component of human sebum lipids. As the distance of double bonds from the carboxyl group increases, the expected fragmentation patterns for the double bonds emerge as marked.
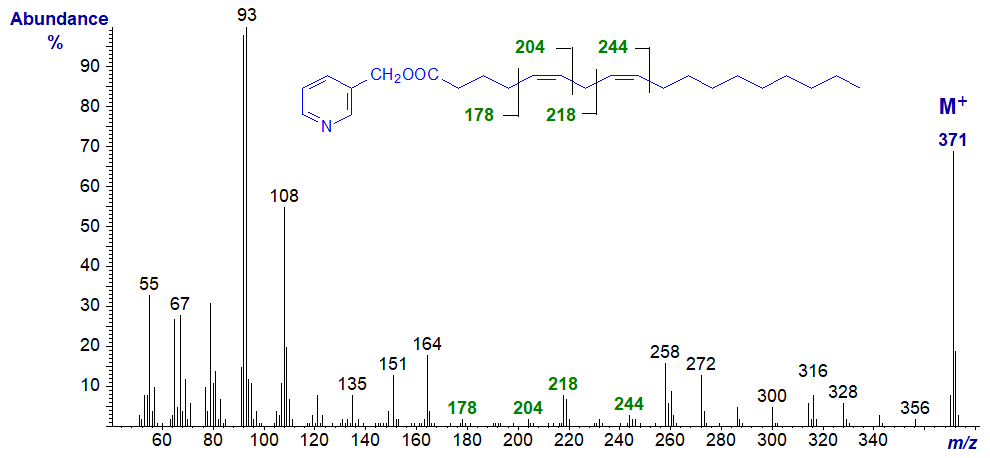
3-Pyridylcarbinyl 6,9-octadecadienoate (6,9-18:2). In this and many of the subsequent spectra in this series, as I have high-lighted, the double bonds are easily located and little further comment is necessary.
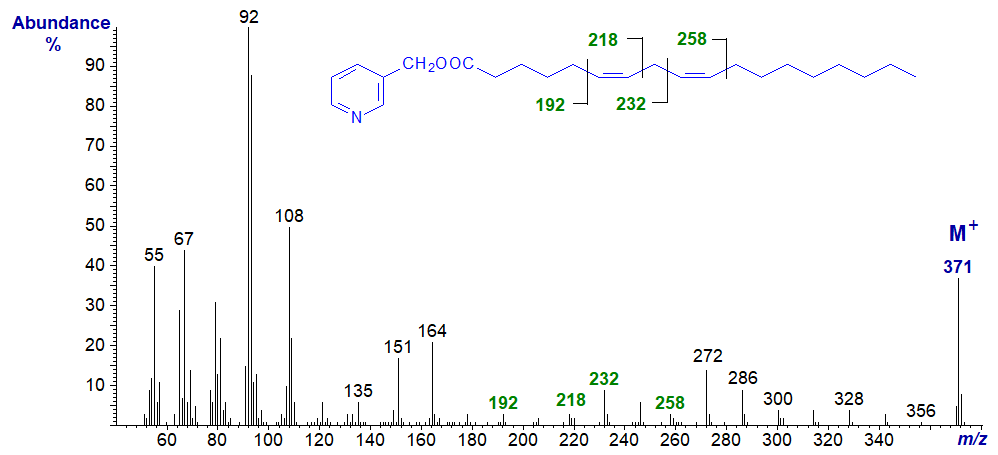
3-Pyridylcarbinyl 7,10-octadecadienoate (7,10-18:2).
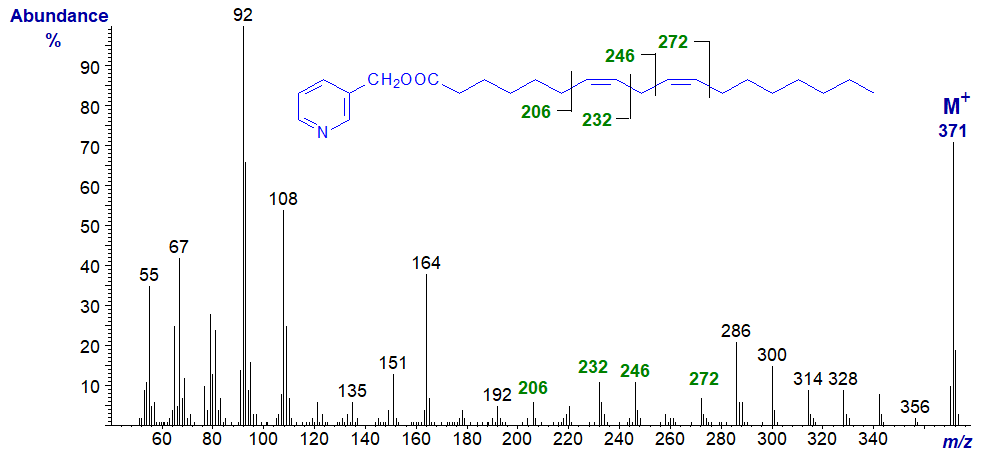
3-Pyridylcarbinyl 8,11-octadecadienoate (8,11-18:2) -
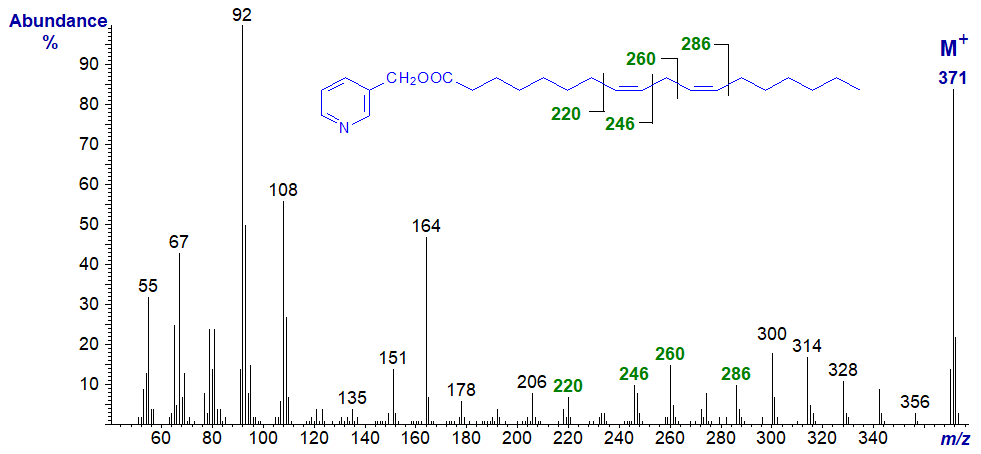
3-Pyridylcarbinyl 9,12-octadecadienoate - see the beginning of this web page (Figure 1).
3-Pyridylcarbinyl 10,13-octadecadienoate (10,13-18:2) -
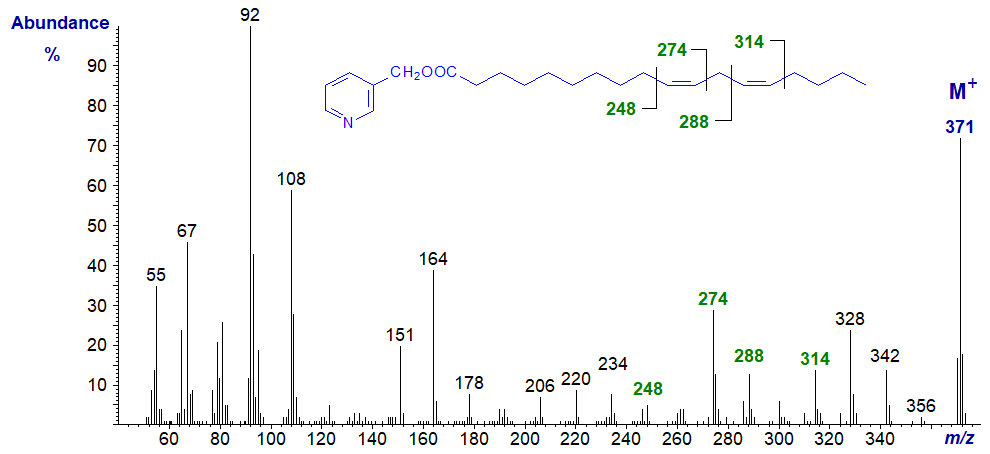
3-Pyridylcarbinyl 11,14-octadecadienoate (11,14-18:2) (Christie et al., 1987) -
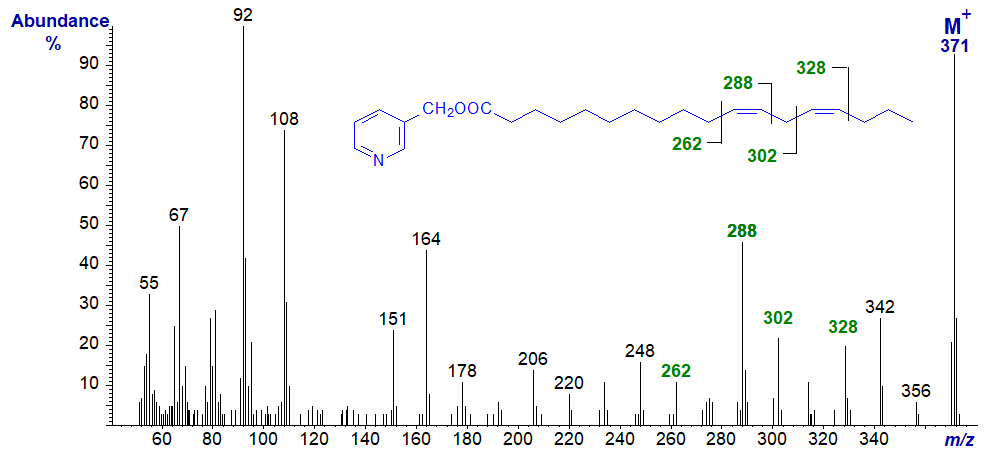
3-Pyridylcarbinyl 12,15-octadecadienoate (12,15-18:2) -
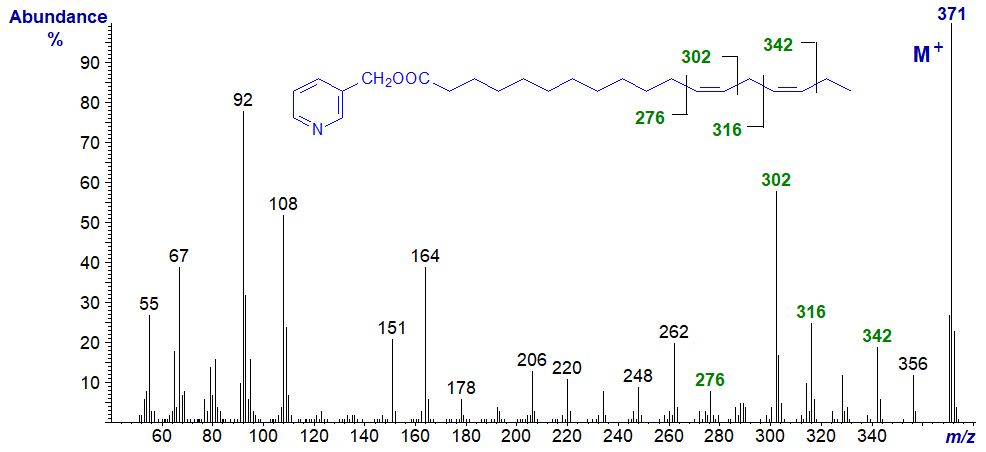
3-Pyridylcarbinyl 13,16-octadecadienoate (13,16-18:2) -
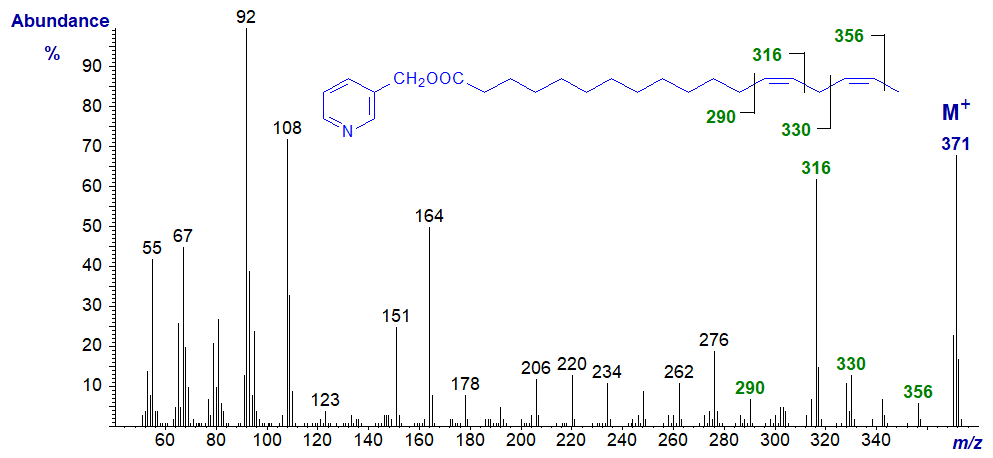
3-Pyridylcarbinyl 14,17-octadecadienoate (14,17-18:2). It is possible even with this last of the isomers to locate the double bonds by the specific fragments marked, especially if we look for the gaps of 40 amu as well as those of 26 amu.
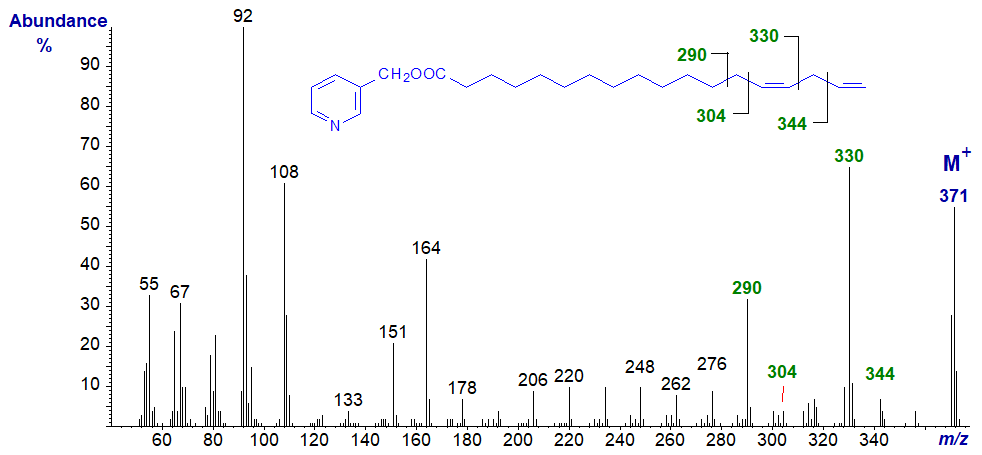
Spectra of 3-pyridylcarbinol esters of many more dienoic fatty acids of this type of differing chain lengths are illustrated in our Archive section, but without interpretation. Many of these have not been formally published elsewhere.
Some other Methylene-Interrupted Dienes
Mass spectra of 3-pyridylcarbinol esters of methylene-interrupted dienes of different chain-lengths are of course interpreted in the same way as the next two examples show. The spectrum of 3-pyridylcarbinyl 7,10-heptadecadienoate (7,10-17:2) is -
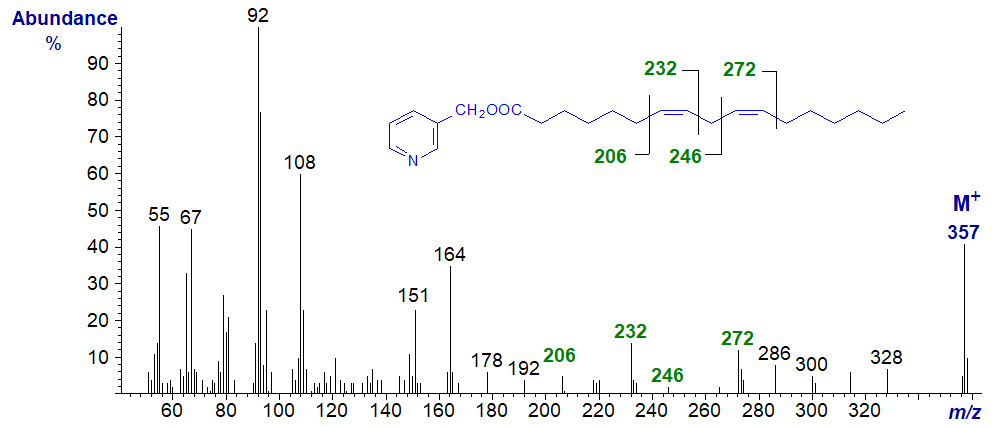
The diagnostic ion for each of the double bonds are as illustrated and are the same as for 7,10-18:2 isomer above.
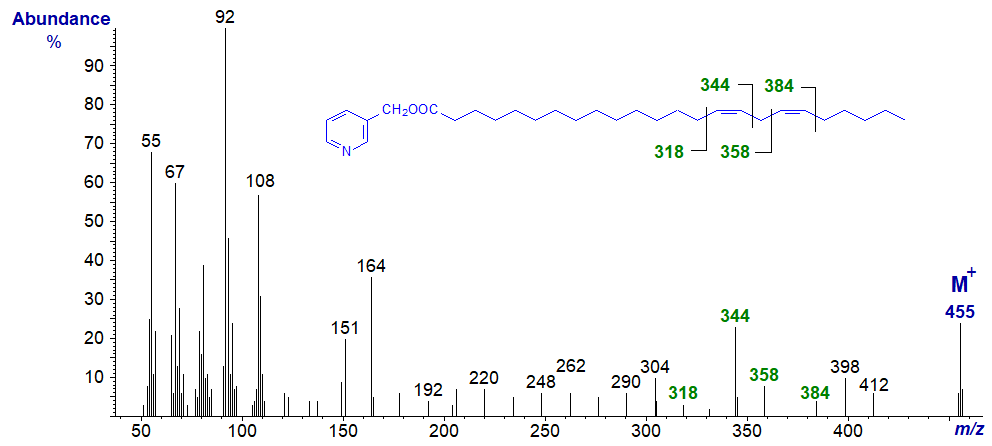
Similarly, in the mass spectrum of 3-pyridylcarbinyl 15,18-tetracosadienoate (15,18-24:2), the important diagnostic ions are at m/z = 318 and 344 for the double bond in position 15 and at 358 and 384 for that in position 18. There is of course no equivalent 18:2 isomer.
Spectra of 3-pyridylcarbinol esters of many more dienoic fatty acids are illustrated in our Archive section, but without interpretation. Many of these have not been published formally elsewhere.
References
- Christie, W.W. and Holman, R.T. Synthesis and characterization of the complete series of methylene-interrupted cis,cis-octadecadienoic acids. Chem. Phys. Lipids, 1, 407-423 (1967); DOI.
- Christie, W.W., Brechany, E.Y. and Holman, R.T. Mass spectra of the picolinyl esters of isomeric mono- and dienoic fatty acids. Lipids, 22, 224-228 (1987); DOI.
- Harvey, D.J. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom., 9, 33-38 (1982); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
