Mass Spectrometry of DMOX Derivatives
Dienoic Fatty Acids. Part 2. Conjugated and
Bis- and Polymethylene-Interrupted Dienes
In this and the previous web page, the electron-impact mass spectra of DMOX derivatives of those dienoic fatty acids that are most likely to be encountered in nature, together with some useful model compounds, are described. Here in Part 2, the spectra of DMOX derivatives of dienes with conjugated double bond systems and others with two or more methylene groups between the double bonds are discussed. Part 1 describes the spectra of the DMOX derivatives of methylene-interrupted dienoic acids. Most of the spectra illustrated here have not been published elsewhere in the scientific literature to my knowledge.
When the first double bond is close to the carboxyl group, the general fragmentation rule for double bond location is no longer appropriate, and the 'fingerprint' spectrum for the relevant monoene derivative is an invaluable guide to identification.
Conjugated Dienoic Fatty Acids
DMOX derivatives appear to be better than 3-pyridylcarbinol ('picolinyl') esters (or pyrrolidides) for characterization of fatty acids with conjugated double bond systems (see Mass spectra of 3-pyridylcarbinol esters. Dienoic fatty acids), although MTAD adducts of methyl esters are also valuable (more here ...), as is the use of acetonitrile-chemical-ionization mass spectrometry (though we have no personal experience of the last technique). DMOX derivatives usually give spectra that are interpretable in terms of double bond positions when isomers are well resolved on the GC column, although it is always helpful if authentic standards or standard spectra are available for comparison. The spectrum of the DMOX derivative of 9‑cis,11-trans-octadecadienoate (9,11-18:2) follows (Spitzer et al., 1994) -
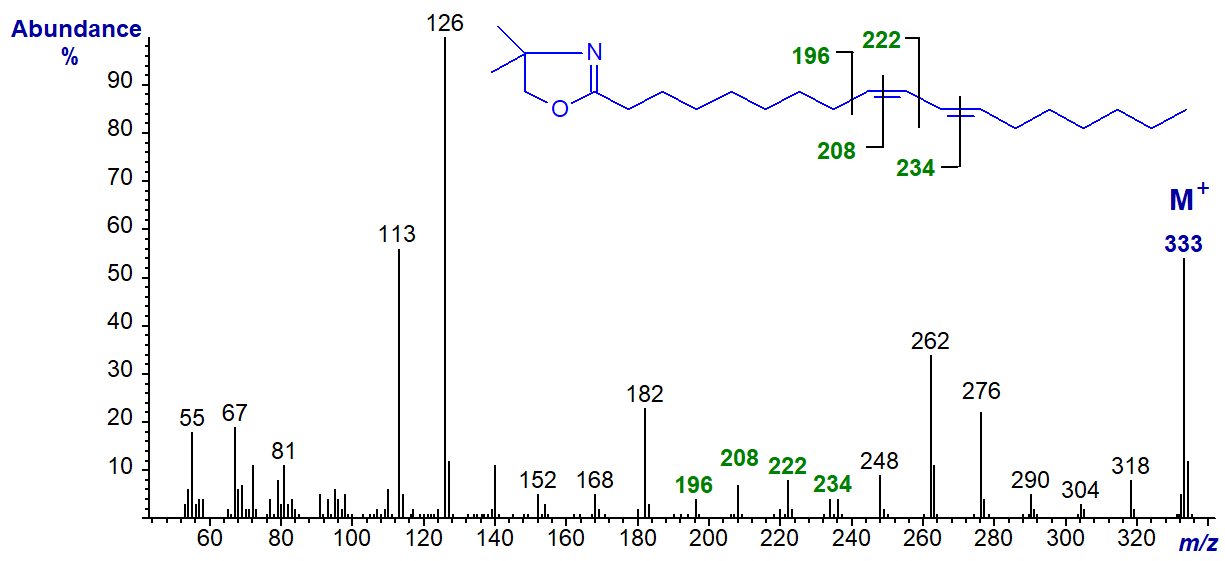
The molecular ion is relatively abundant, and the positions of the double bonds are given by the gaps of 12 amu between m/z = 196 and 208 and between 222 and 234, for the double bonds in positions 9 and 11, respectively. The prominent ions formed by rearrangements involving formation of further conjugated intermediates are diagnostic, i.e., at m/z = 262 and 276, and these have even been used to quantify complex mixtures of isomers in commercial conjugated linoleic acid preparations by means of selective ion monitoring.
The DMOX derivative of 10-trans,12-cis-octadecadienoate (10,12-18:2) -
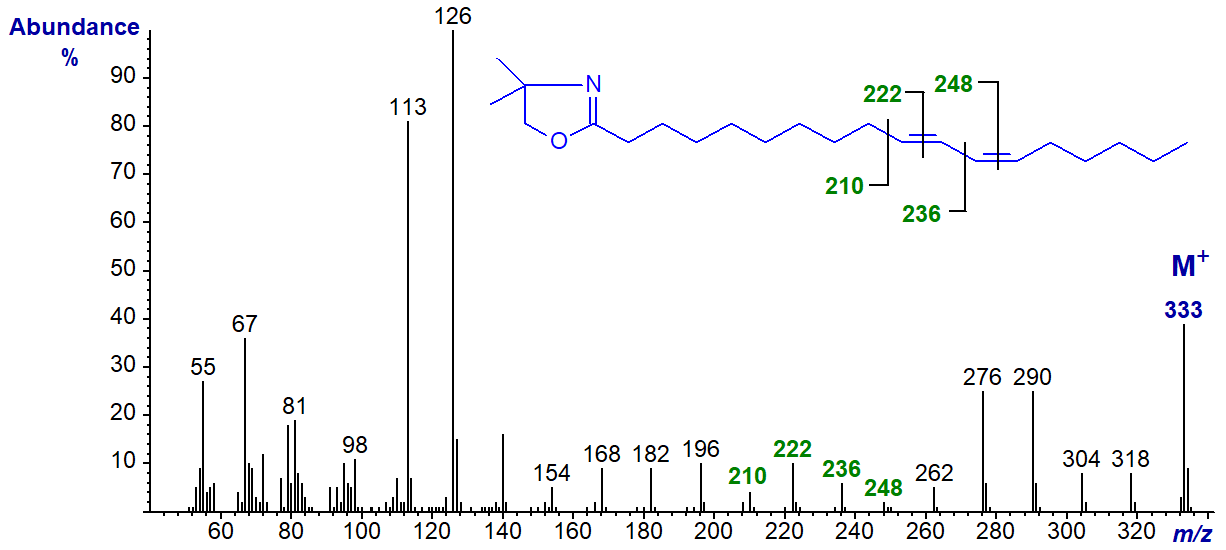
In this instance, the diagnostic ions are shifted up by 14 amu in comparison to the previous.
Bis-Methylene-Interrupted Dienoic Fatty Acids
It is becoming apparent that bis- and polymethylene-interrupted dienoic fatty acids are more common in nature than may have been supposed, and fatty acids with a 5,9-double bond system or their chain elongation products are typical components of seed oils from Gymnosperms or of certain marine invertebrates such as sponges. The spectra of DMOX derivatives of 5,9-18:2 and of some other natural fatty acids encountered during our research are illustrated below.
The DMOX derivative of 5,9-octadecadienoate (5,9-18:2) from Pinus contorta seed oil -
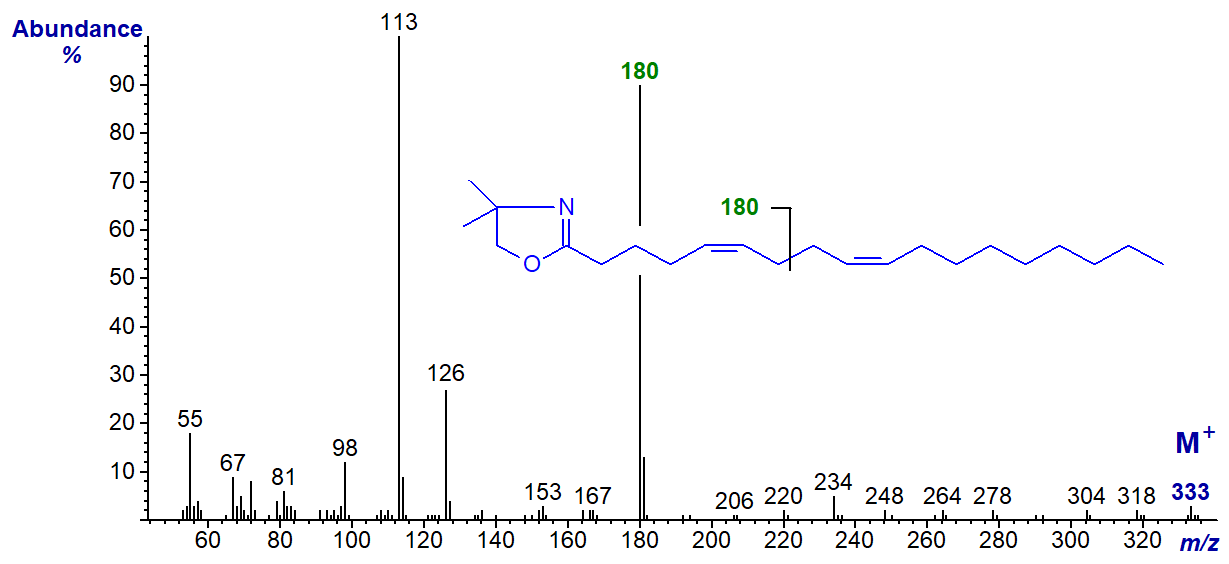
Although it is not possible to locate gaps of 12 amu that define the position of the double bonds, this hardly matters as the abundant ion at m/z = 180 for cleavage at the centre of the bis-methylene-interrupted double bond system is sufficiently characteristic (Wolff and Christie, 2002).
The DMOX derivative of 7,11-octadecadienoate (7,11-18:2) from the sponge Haliclona cinerea -
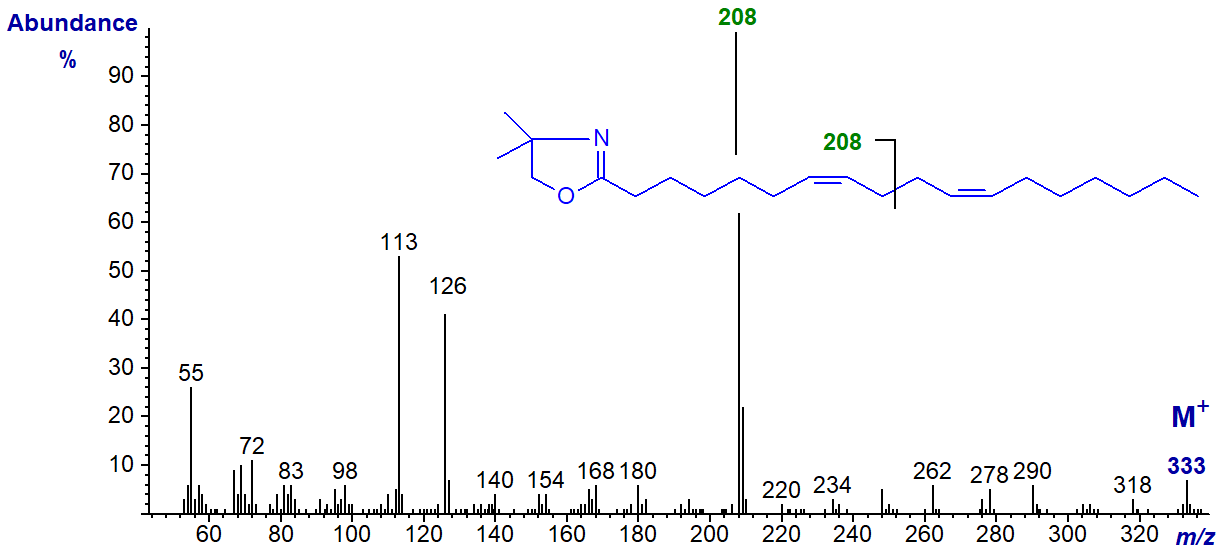
In this instance, the key diagnostic ion has shifted upwards as expected to m/z = 208.
As the double bonds move further from the carboxyl group additional diagnostic ions for each double bond become apparent. For example, the DMOX derivative of 9,13-eicosadienoate (9,13-20:2) from the sponge Haliclona cinerea -
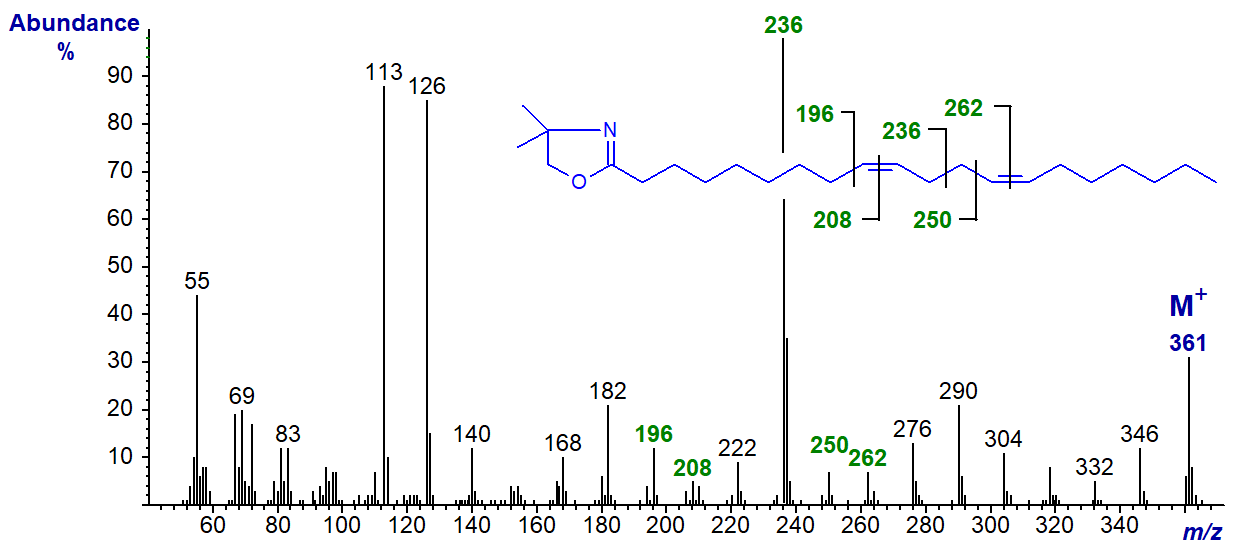
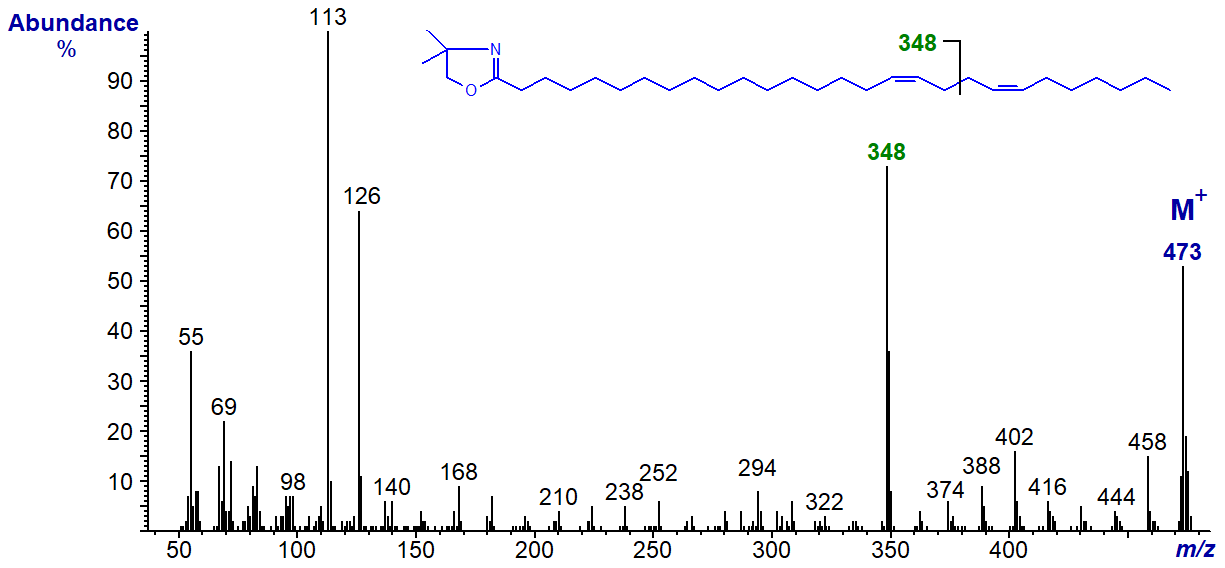
The main diagnostic ion has shifted upwards as expected to m/z = 236, and further diagnostic ions for the double bonds locate gaps of 12 amu between m/z = 196 and 208 and 250 and 262 for positions 9 and 13, respectively. The main diagnostic ion is still highly distinctive even in the spectrum of the DMOX derivative of 17,21-octacosadienoate (17,21-28:2) at m/z = 348 -
Polymethylene-Interrupted Dienoic Fatty Acids
Fatty acids with more than two methylene groups between double bonds are perhaps less common in nature, but some mass spectra of DMOX derivatives of natural fatty acids of this type are illustrated below. With such compounds, as mentioned briefly above, the first double bond is located in the same way as for isolated monoenes when it is close to the carboxyl group, and recourse to comparison with authentic spectra is essential.
The DMOX derivative of trans-3,cis-9-octadecadienoate (3,9-18:2), an occasional minor component in seed oils of the Compositae, has the next spectrum -
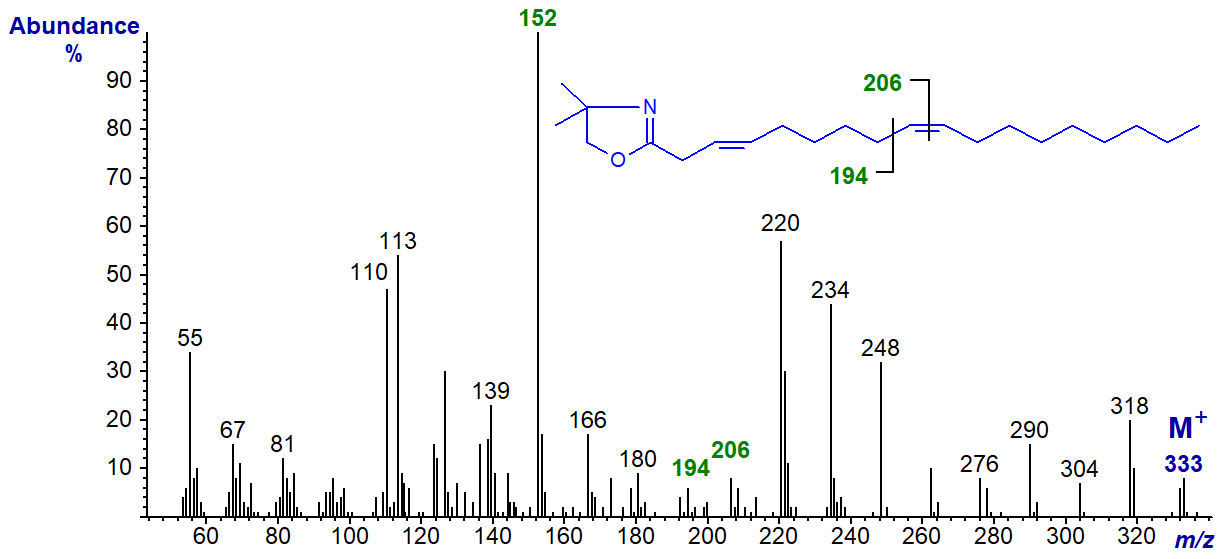
The double bond in position 3 is recognized by the diagnostic ion at m/z = 152, while that in position 9 is located by the gap of 12 amu between m/z = 194 and 206. With the latter, the gap of 40 amu between m/z = 180 and 220 is a further useful guide. On the other hand, it is possible that the first double bond has isomerized to position 2 under the alkaline derivatization conditions. The configuration of the double bond in position 3 must be confirmed by means other than electron-impact mass spectrometry.
The DMOX derivative of 5,11-eicosadienoate (5,11-20:2) from Pinus contorta seed oil -
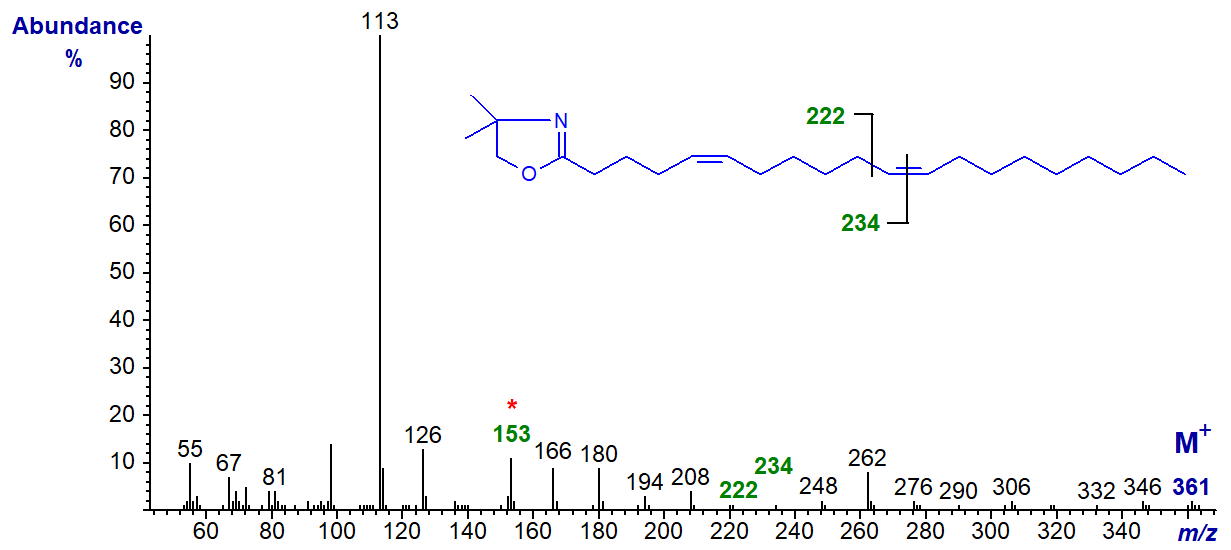
The double bond in position 5 is recognized by the diagnostic ion at m/z = 153, while that in position 11 is located by the gap of 12 amu between m/z = 222 and 234 (Wolff and Christie, 2002). All the ions in the high mass range tend to be of low abundance with 5-isomers.
The DMOX derivative of 5,13-docosadienoate (5,13-22:2) from meadowfoam oil. In this instance, the double bond in position 13 is indicated by small but distinct ions at m/z = 250 and 262.
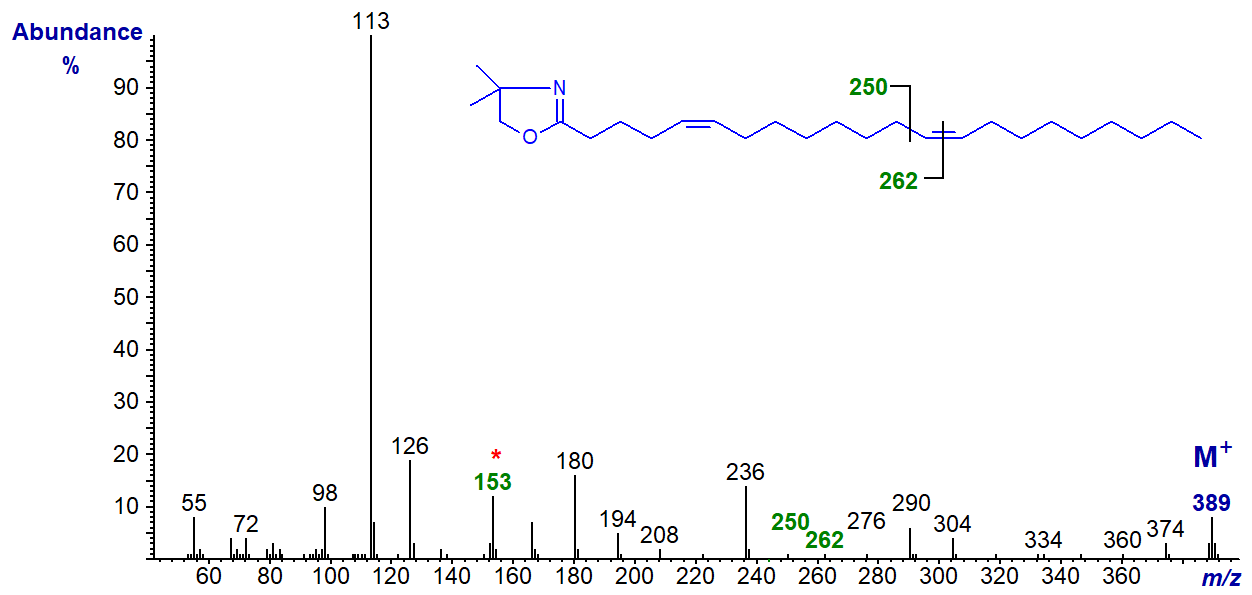
The DMOX derivative of 7,13-docosadienoate (7,13-22:2) from a marine invertebrate (Rapana thomasiana). In this and the next spectrum, diagnostic ions are marked with no further comment.
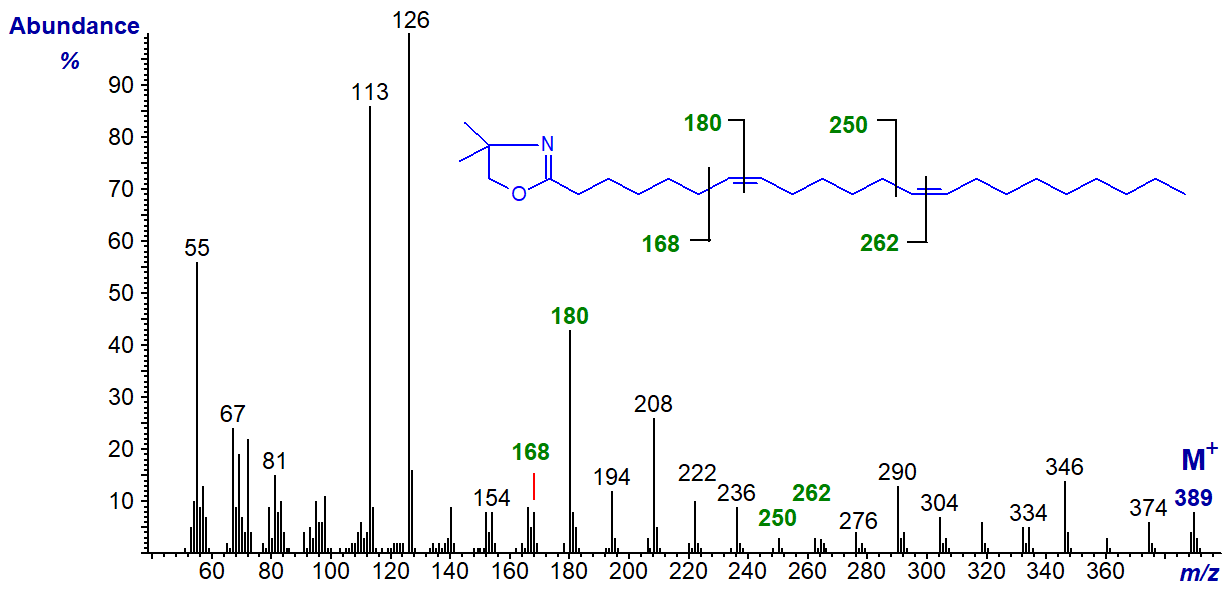
The DMOX derivative of 7,15-docosadienoate (7,15-22:2) is from the same source as the previous spectrum (this spectrum was published first by Dunstan et al., 1993).
We have unpublished mass spectra of the DMOX derivatives of many more bis- and poly-methylene-interrupted dienoic fatty acids with a range of chain-lengths (including some branched-chain isomers), and these are available in the Archive section of these web pages but without interpretation.
References
- Dunstan, G.A., Volkman, J.K. and Barrett, S.M. The effect of lyophilization on the solvent extraction of lipid classes, fatty acids and sterols from the oyster Crassostrea gigas. Lipids, 28, 937-944 (1993); DOI.
- Spitzer, V., Marx, F. and Pfeilsticker, K. Electron impact mass spectra of the oxazoline derivatives of some conjugated diene and triene C18 fatty acids. J. Am. Oil Chem. Soc., 71, 873-876 (1994); DOI.
- Wolff, R.L. and Christie, W.W. Structures, practical sources (gymnosperm seeds), gas-liquid chromatographic data (equivalent chain lengths), and mass spectrometric characteristics of all-cis 5-olefinic acids. Eur. J. Lipid Sci. Technol., 104, 234-244 (2002); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
