Mass Spectrometry of 3-Pyridylcarbinol Esters
Allenic Fatty Acids
 Fatty acids
with allenic fatty acid systems are rather uncommon in nature, and we have not encountered them in our work.
However, we do have spectra from a series of synthetic allenes, both as the 3-pyridylcarbinol ('picolinyl') and methyl esters.
As these spectra are unique and have relevance to the interpretation of the spectra of acetylenic fatty acids,
some representatives are illustrated with a minimal commentary.
Further details are available in the published paper, but more spectra can be illustrated here than is possible in a printed publication
(Christie, W.W., Brechany, E.Y., Lie Ken Jie, M.S.F. and Wong, C.F.
Mass spectrometry of derivatives of isomeric allenic fatty acids.
Biol. Mass Spectrom., 21, 267-270 (1992); DOI).
Here the spectra of 3‑pyridylcarbinol esters are discussed, while those of
methyl esters are described in a separate document.
There is an account of the natural occurrence of allenic fatty acids in the
Lipid Essentials section of this website.
Fatty acids
with allenic fatty acid systems are rather uncommon in nature, and we have not encountered them in our work.
However, we do have spectra from a series of synthetic allenes, both as the 3-pyridylcarbinol ('picolinyl') and methyl esters.
As these spectra are unique and have relevance to the interpretation of the spectra of acetylenic fatty acids,
some representatives are illustrated with a minimal commentary.
Further details are available in the published paper, but more spectra can be illustrated here than is possible in a printed publication
(Christie, W.W., Brechany, E.Y., Lie Ken Jie, M.S.F. and Wong, C.F.
Mass spectrometry of derivatives of isomeric allenic fatty acids.
Biol. Mass Spectrom., 21, 267-270 (1992); DOI).
Here the spectra of 3‑pyridylcarbinol esters are discussed, while those of
methyl esters are described in a separate document.
There is an account of the natural occurrence of allenic fatty acids in the
Lipid Essentials section of this website.
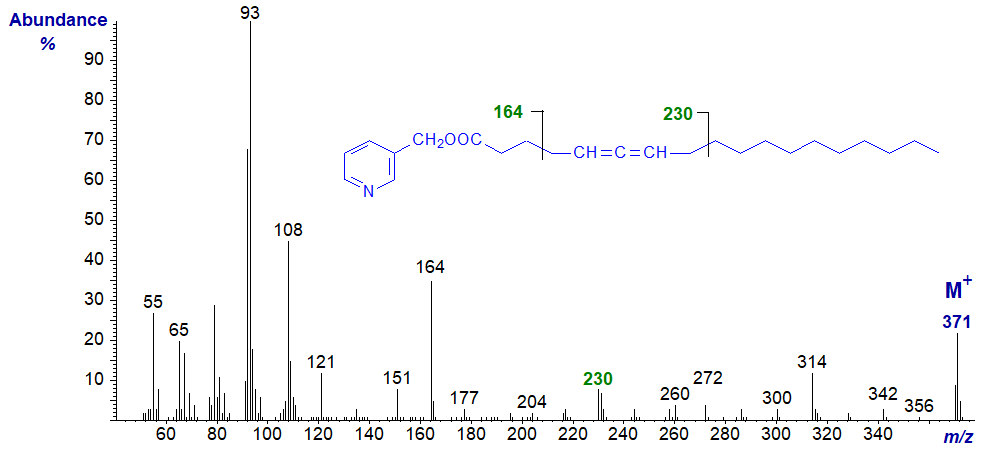
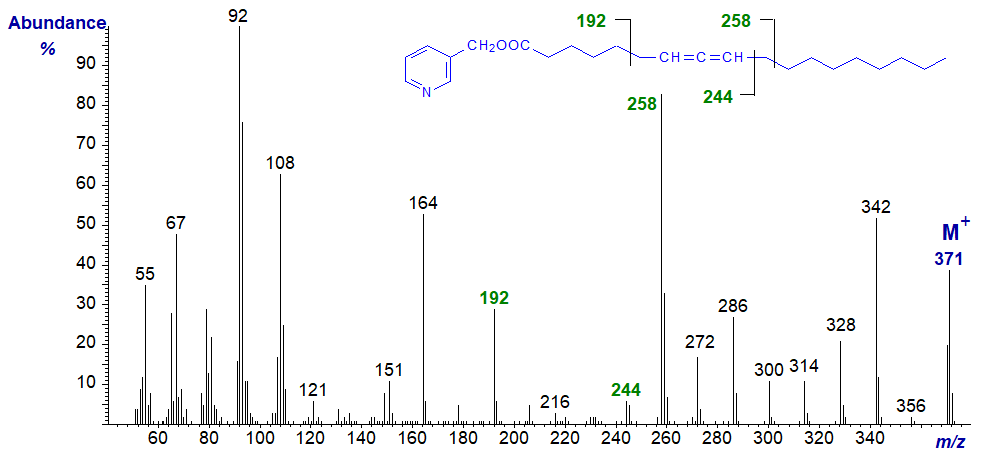
There is always a distinctive molecular ion, together with the expected ions from the carboxyl end at m/z = 92/93, 108, 151 and 164 (see the web page on the corresponding saturated derivatives). Interpretation of the location of the allenic group is not straightforward in all instances, but the main diagnostic fragmentations are beta to the allenic moiety on each side, as illustrated simplistically here, but with little further discussion.
Mass spectrum of 3-pyridylcarbinyl 5,6-octadecadienoate -
Mass spectrum of 3-pyridylcarbinyl 7,8-octadecadienoate -
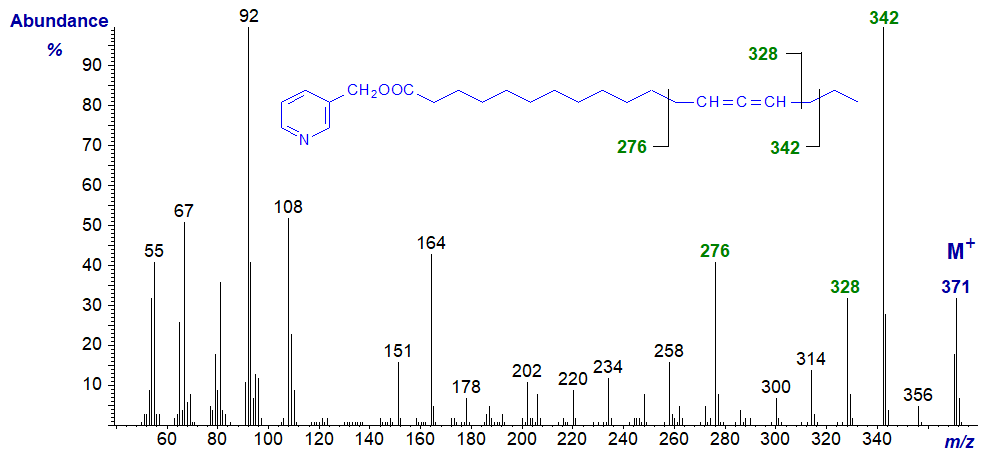
Mass spectrum of 3-pyridylcarbinyl 9,10-octadecadienoate. Note that from this isomer onwards, cleavage alpha to the double bond system on the distal side (at m/z = 272 in this instance) becomes more prominent.
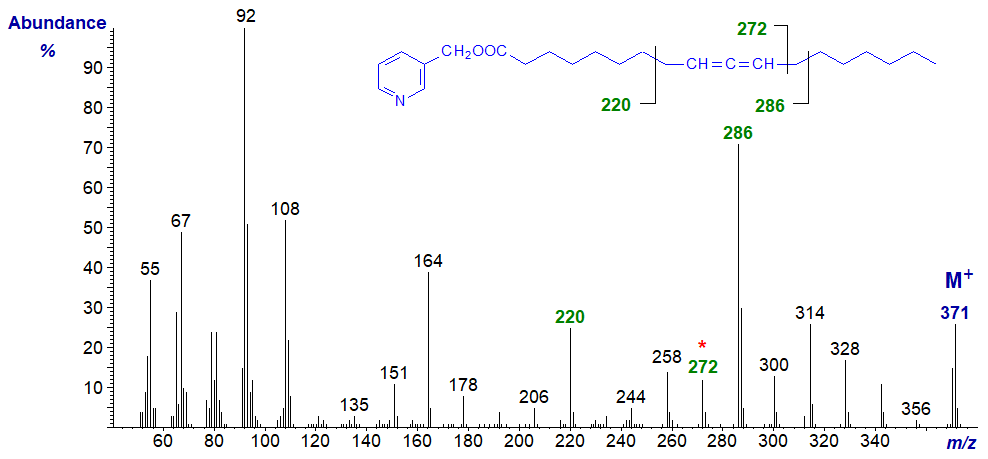
As the double bond system moves further from the carboxyl group, rearrangement ions within the double bond system stand out more, e.g., that at m/z = 258 in this example.
Mass spectrum of 3-pyridylcarbinyl 11,12-octadecadienoate -
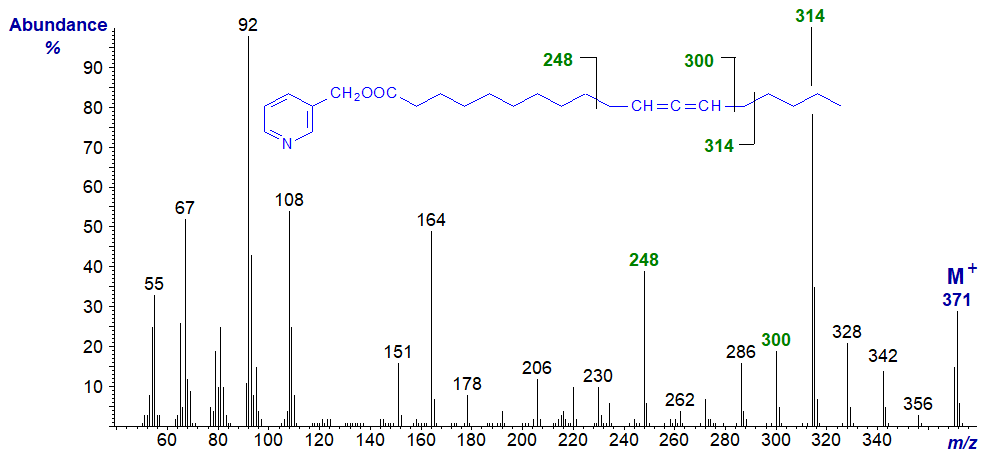
Mass spectrum of 3-pyridylcarbinyl 13,14-octadecadienoate -
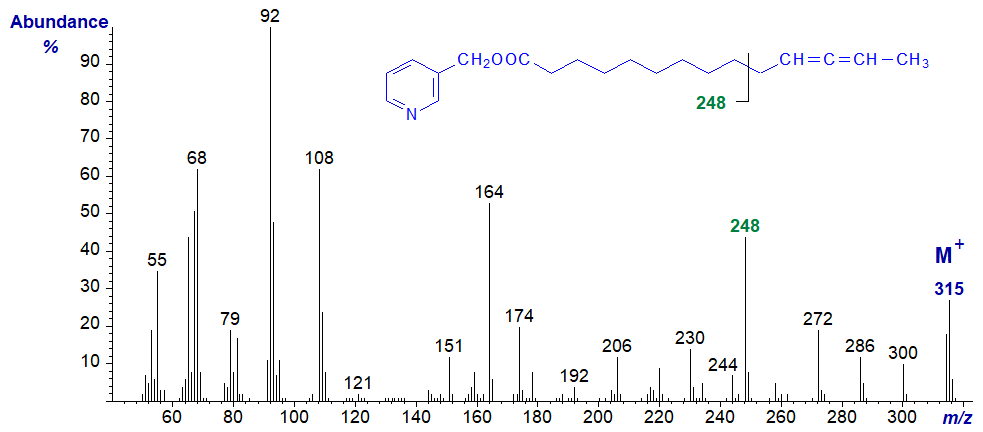
When the allenic system is in the penultimate position as in the spectrum of 3-pyridylcarbinol 11,12-tetradecadienoate, the ion on the carboxyl side of the allene group, at m/z = 248 in this instance, is diagnostic (cf., the spectrum of the 11,12-18:2 derivative above) -
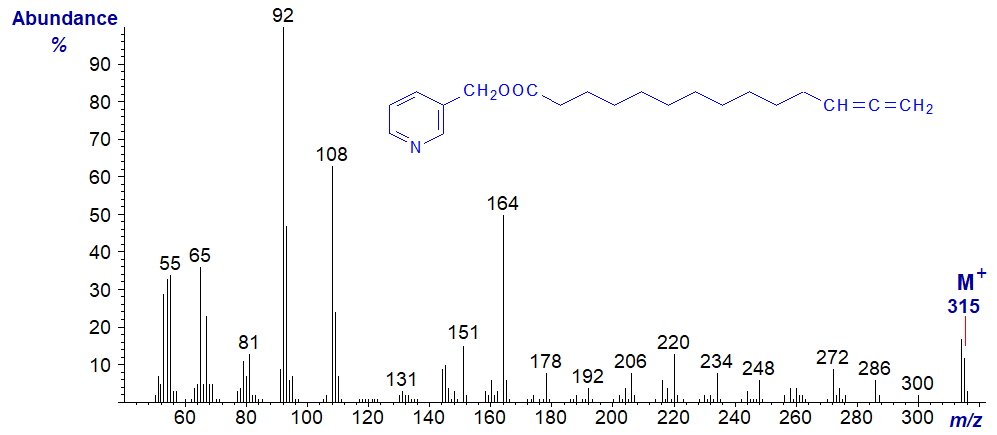
When the allenic system is terminal as in the spectrum of 3-pyridylcarbinol 12,13-tetradecadienoate, any detailed interpretation is impossible, and we must simply treat it as a fingerprint. This is a failing of all nitrogen-containing derivatives with unsaturation of all kinds in the terminal position.
Attempts to prepare the 3-pyridylcarbinol ester of a 3,4-dienoate isomer were not successful. We have mass spectra on file for 3‑pyridylcarbinol esters of further allenic fatty acids, and these can be found in our Archive page, but without interpretation.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
