Mass Spectrometry of 3-Pyridylcarbinol Esters
Saturated Branched-Chain Fatty Acids
3-Pyridylcarbinol ('picolinyl') esters are especially useful for structure determination of branched-chain fatty acids, especially the iso- and anteiso-methyl-branched forms, i.e., those most likely to be encountered in natural samples, where DMOX derivatives are less suitable. Our web page on mass spectrometry of 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). An early review article by D.J. Harvey (1992) is still very valuable. All the spectra illustrated were obtained from natural samples during my own research work and is as comprehensive selection as you will find anywhere, although there are some obvious gaps. Most of these spectra will not have been illustrated elsewhere.
Iso- and anteiso-Methyl-Branched Fatty Acids
Most naturally occurring monomethyl-branched fatty acids have saturated alkyl chains, and the most common of these are the iso- and anteiso-methyl isomers. They are produced by bacteria mainly, but they can enter animal tissues via the food chain (see our web page on this topic here..). The mass spectrum of 3‑pyridylcarbinyl iso-methyl-octadecanoate (17‑methyl‑18:0) follows (Harvey, 1982) -
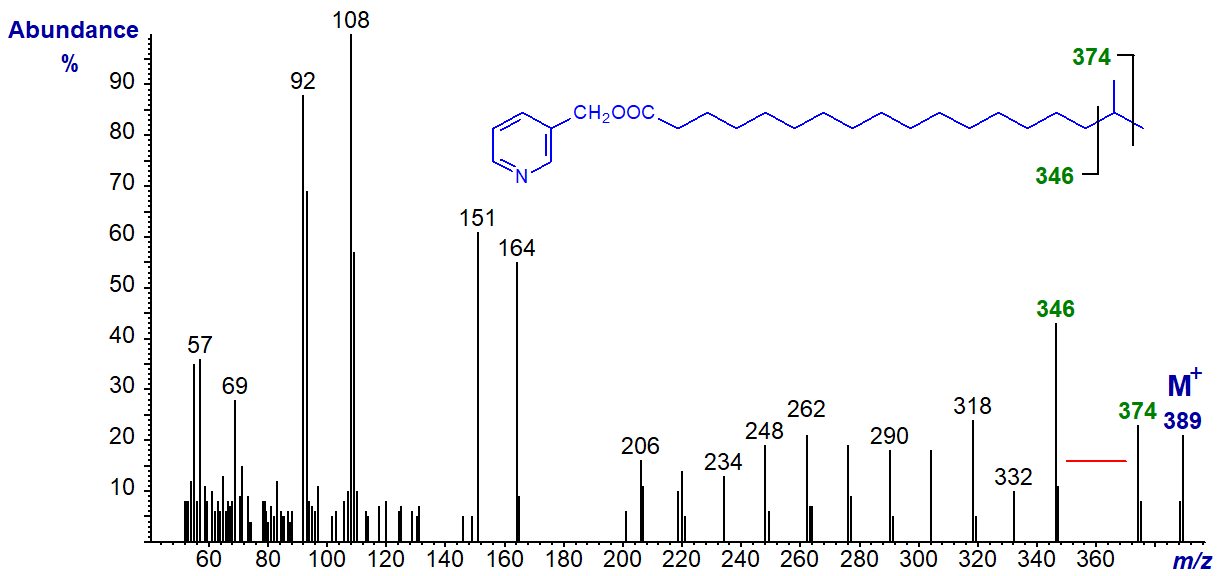
The spectrum resembles that of a straight-chain saturated fatty acid except for the obvious gap of 28 amu between m/z = 346 and 374, which represents loss of carbon-17 and its attached methyl group, locating it definitively. The remaining ions are uniformly 14 amu apart. This is one example where 3‑pyridylcarbinol esters have a distinct advantage over other types of derivative, as with this and the next spectrum there are no 'noise' peaks in the gap.
In the mass spectrum of 3-pyridylcarbinyl anteiso-methyl-octadecanoate (16-methyl-18:0), this gap of 28 amu is shifted to between m/z = 332 and 360 as -
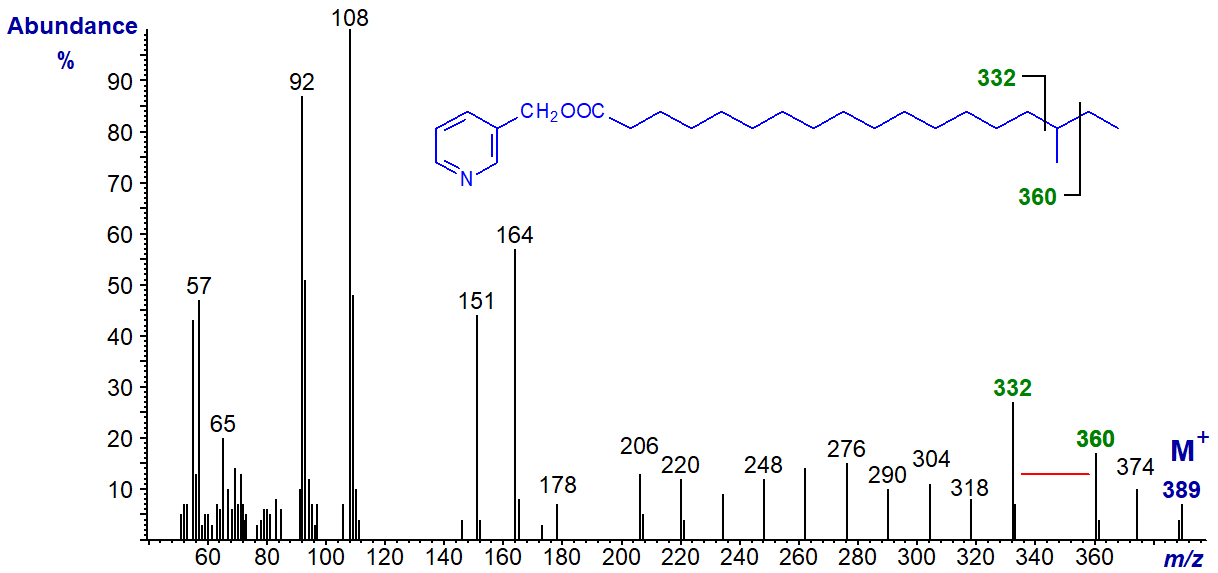
Fatty Acids with Centrally Located Methyl Branches
The same principles apply for the location of methyl branches in other positions. In the mass spectrum of 3-pyridylcarbinyl 15‑methyloctadecanoate, the gap of 28 amu is shifted to m/z = 318 to 346 as -
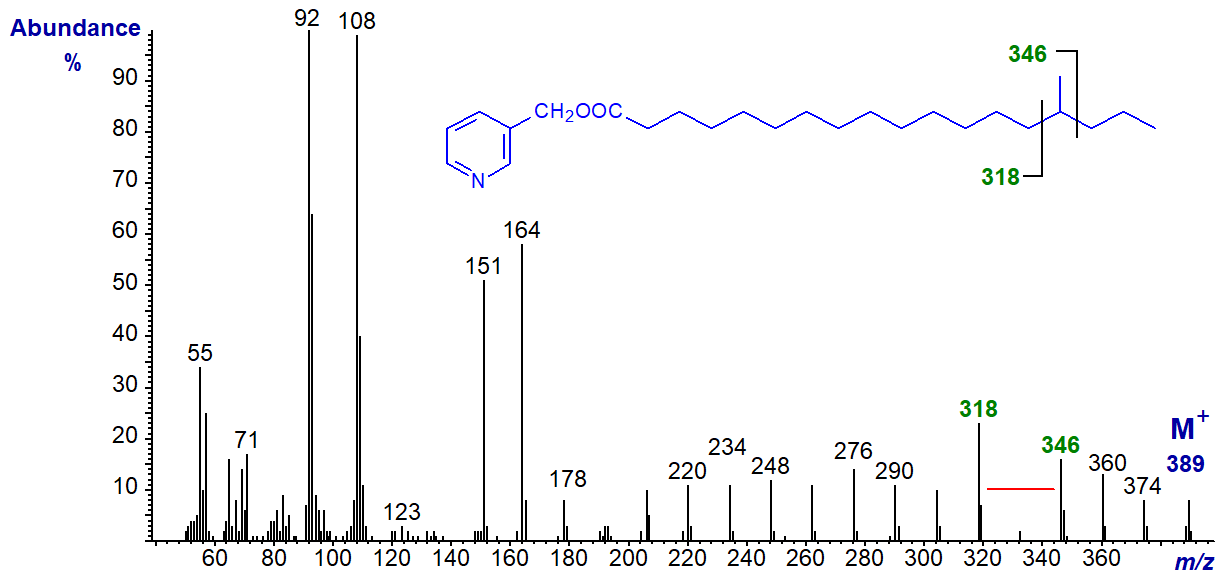
With that of 3-pyridylcarbinyl 11-methyloctadecanoate, the gap for the methyl branch is between m/z = 262 and 290 as -
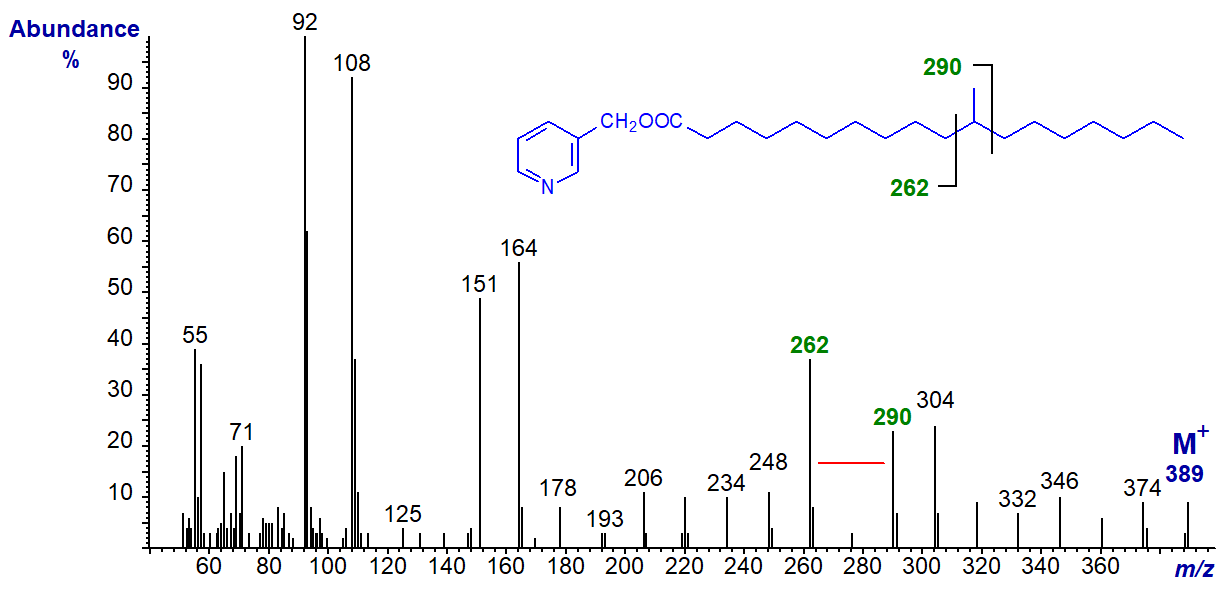
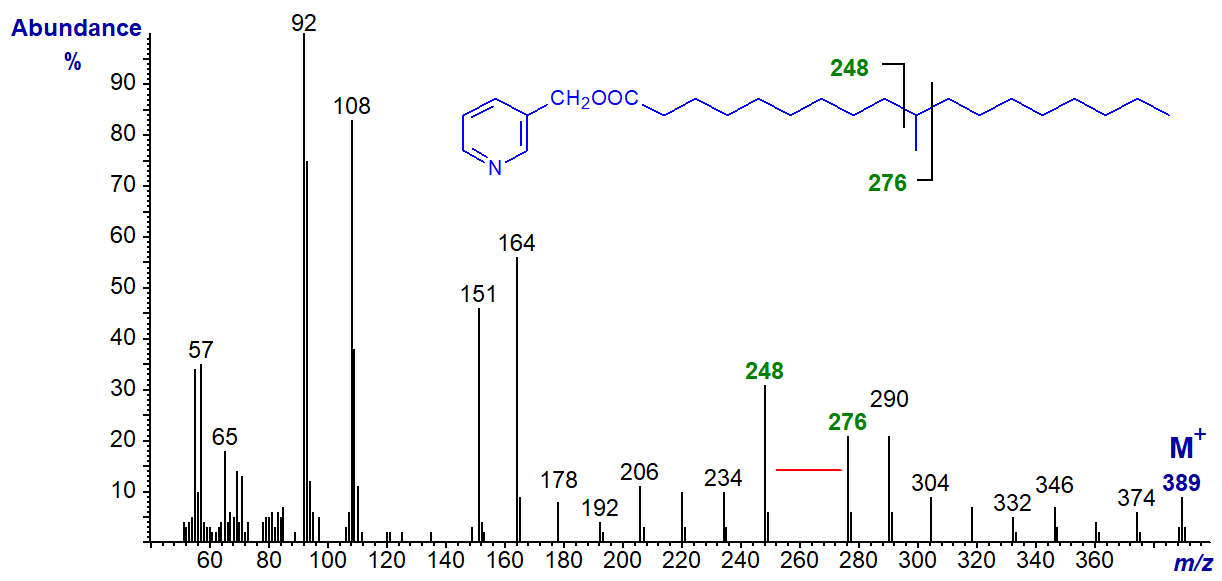
With that of 3-pyridylcarbinyl 10-methyloctadecanoate (tuberculostearate), the gap is between m/z = 248 and 276 -
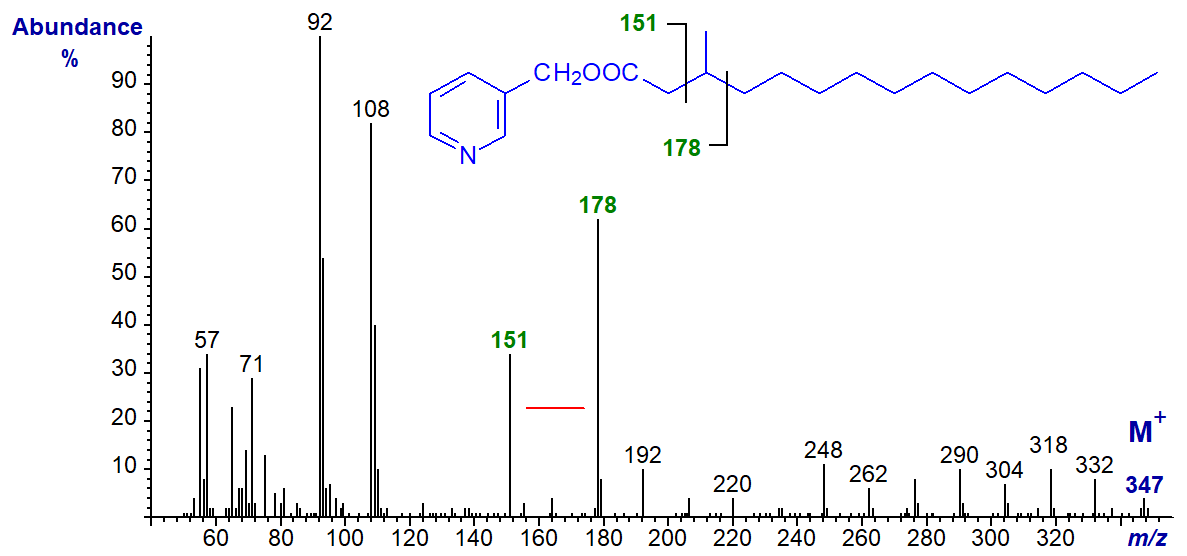
The mass spectrum of 3-pyridylcarbinyl 3-methylpentadecanoate is interesting in that the usual ion at m/z = 164 is shifted to 178, and there is a gap of 27 amu between m/z = 151 and 178 to locate the branch point.
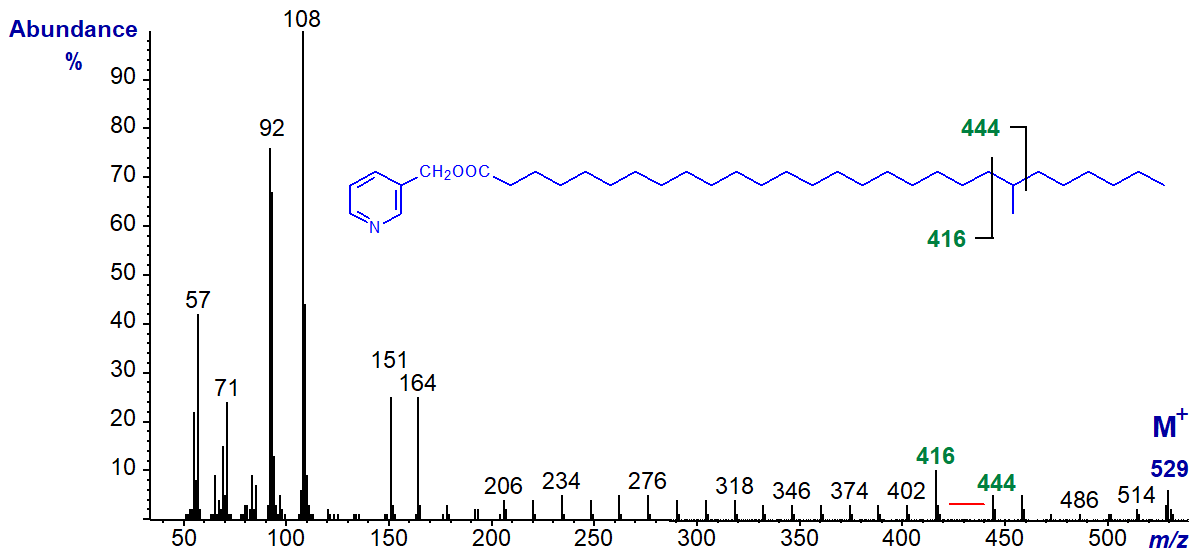
It is also easy to locate methyl-branch points in spectra of very-long-chain fatty acids as with that of 3-pyridylcarbinyl 22-methyl-octacosanoate, as illustrated -
Of course, prediction of the diagnostic features in the spectra of other methyl-branched isomers or homologues not illustrated or archived on this website is straight-forward.
Di- and Polymethyl-Branched-Chain Saturated Fatty Acids
The same principles to the above apply in the interpretation of mass spectra of 3‑pyridylcarbinol esters of di- and polymethyl-branched-chain fatty acids, and the 3‑pyridylcarbinol ester of an unusual dimethyl-branched fatty acid from a sponge, i.e., 8,10-dimethyl-hexadecanoate follows (Nechev et al., 2002).
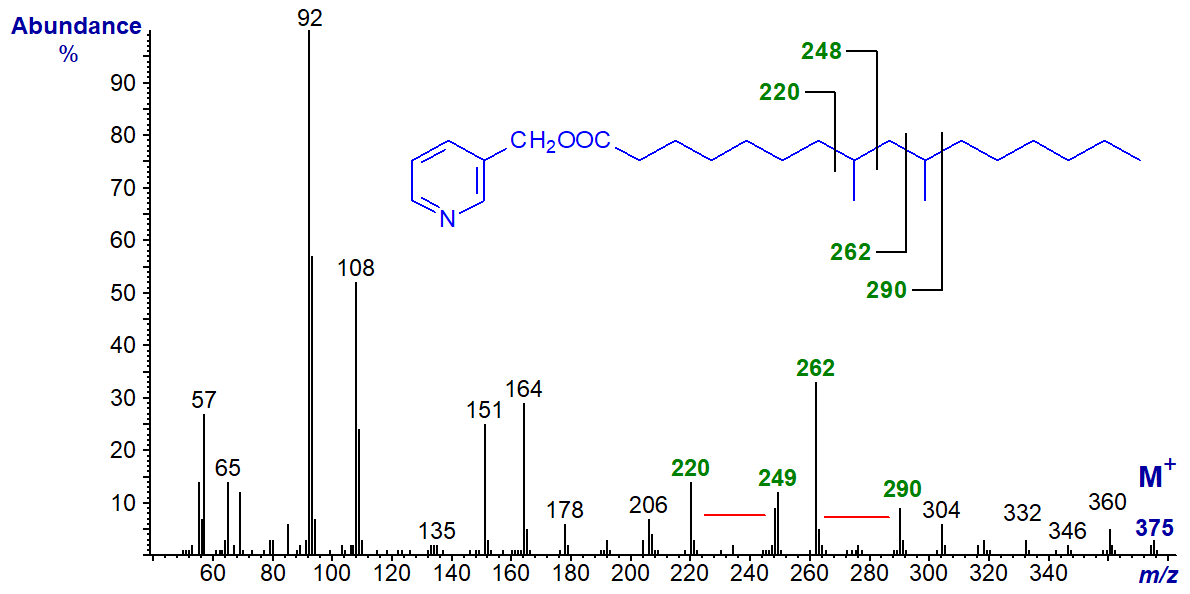
Gaps of 28 amu between m/z = 220 and 248, and between m/z = 262 and 290, locate the branch points.
Polymethyl-branched fatty acids containing isoprene units and derived biosynthetically from phytol are common in nature, but especially as minor components of fish oils or in ruminant tissues, and the spectrum of the 3-pyridylcarbinol ester of 4,8,12-trimethyltridecanoate is -
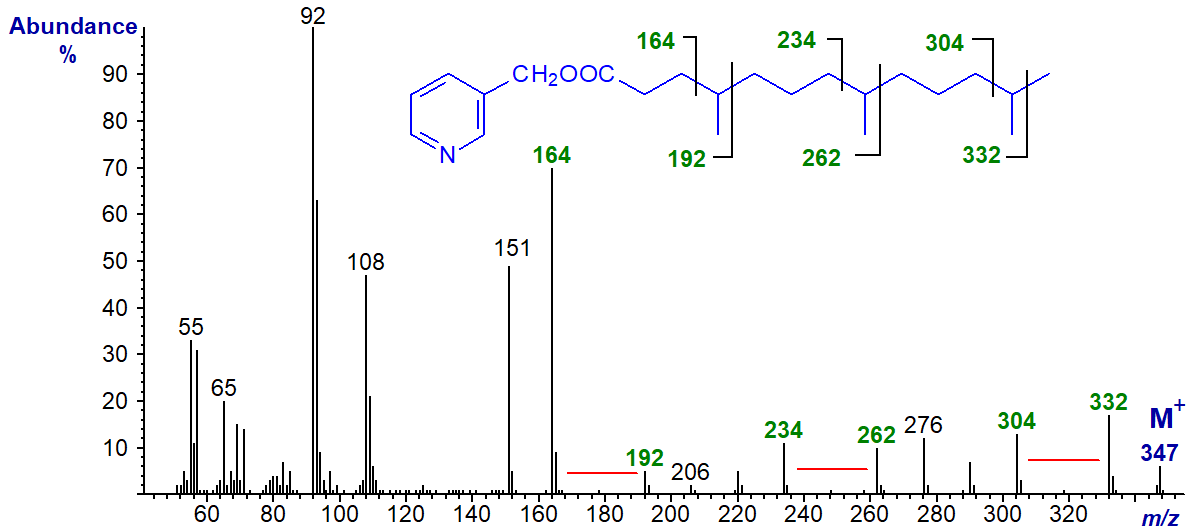
There are clear gaps of 28 amu between ions at m/z = 164 and 192, 234 and 262, and 304 and 332, which locate the methyl groups on carbons 4, 8 and 12, respectively (Christie, 1997).
In the spectrum of the 3-pyridylcarbinol ester of the homologous fatty acid 5,9,13-trimethyl-tetradecanoate, the diagnostic gaps are all 14 amu higher as anticipated -
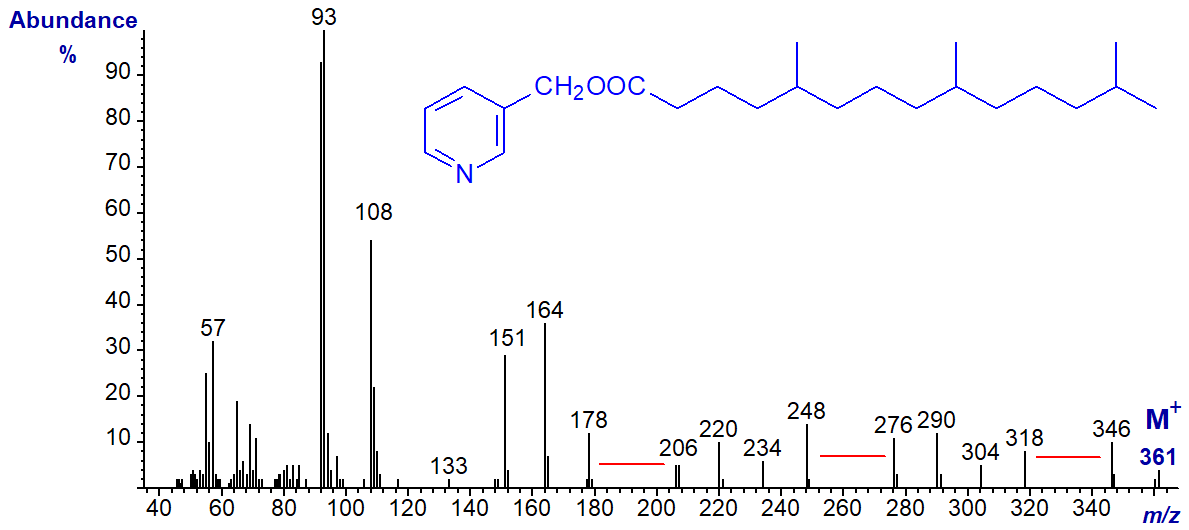
The mass spectrum of 3-pyridylcarbinyl 2,6,10,14-tetramethyl-pentadecanoate (pristanate) has some similar features -
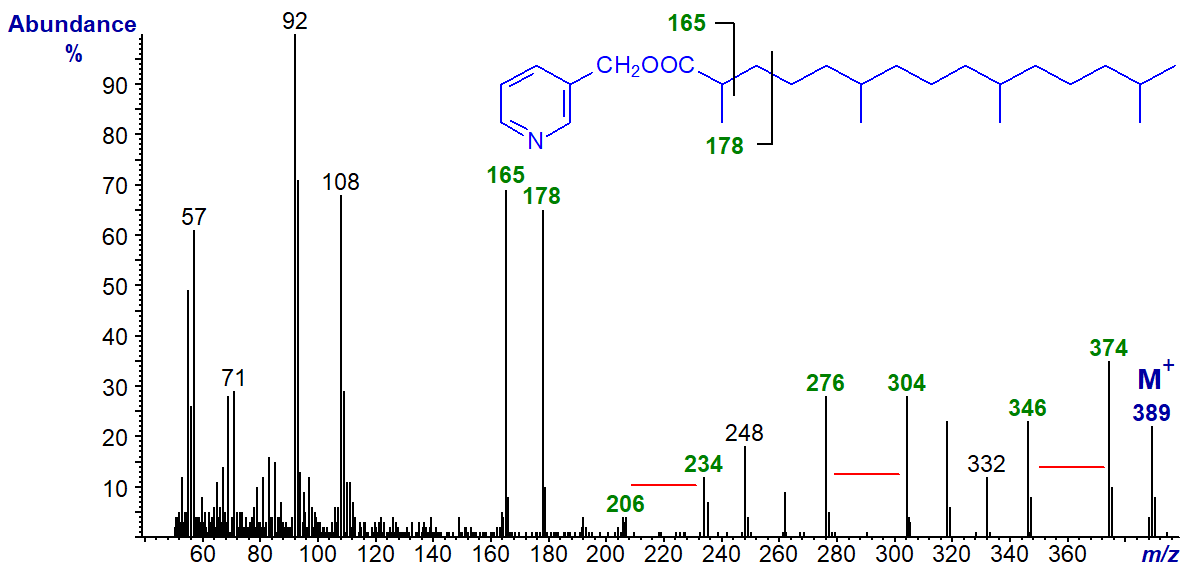
The methyl branches in positions 6, 10 and 14 are located by gaps of 28 amu between m/z = 206 and 234, 276 and 304, and 346 and 374, respectively. The presence of the 2-methyl group is recognized as the ion usually found at m/z = 151 is shifted to 165, while the ion normally at m/z = 164 is shifted to 178.
In comparison, in the spectrum of the homologous 3,7,11,15-tetramethylhexadecanoate (phytanate), the gaps for the methyl branches on carbons 7, 11 and 15 are shifted up by 14 amu as expected.
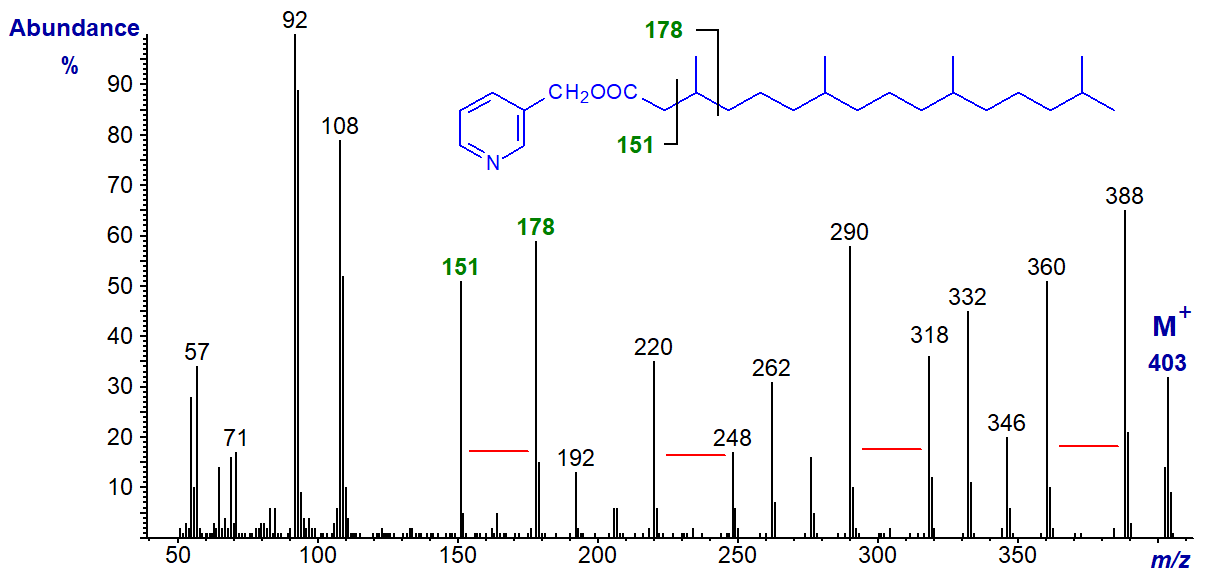
The methyl group on carbon 3 is located by the fact that the ion at m/z = 151 is again substantial, while that normally at m/z = 164 has shifted to 178.
We have spectra of many more 3-pyridylcarbinol esters of branched-chain saturated fatty acids on file, and they can be accessed (but without interpretation) from our Archive page. Only a few of these have been published elsewhere.
References
- Christie, W.W. Structural analysis of fatty acids. In: Advances in Lipid Methodology - Four, pp. 119-169 (edited by W.W. Christie, Oily Press, Dundee) (1997).
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998); DOI.
- Harvey, D.J. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom., 9, 33-38 (1982); DOI.
- Harvey, D.J. Mass spectrometry of picolinyl and other nitrogen-containing derivatives of lipids. In: Advances in Lipid Methodology - One, pp. 19-80 (edited by W.W. Christie, Oily Press, Ayr) (1992).
- Nechev, J., Christie, W.W., Robaina, R., de Diego, F.M., Ivanova, A., Popov, S. and Stefanov, K. Chemical composition of the sponge Chondrosia reniformis from the Canary Islands. Hydrobiologia, 489, 91-98 (2002); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
