Mass Spectrometry of 3-Pyridylcarbinol Esters
Hydroxy Fatty Acids
 We have
a few mass spectra only of 3-pyridylcarbinol ('picolinyl') esters of hydroxy fatty acids available (none of eicosanoids),
and then mainly from standards or well-defined natural sources, so this is a very limited and imperfect review of the topic.
The additional polarity and molecular weight tended to render 3-pyridylcarbinol esters less suitable
for the analysis of complex mixtures (our research interest at one time) by gas chromatography-mass spectrometry.
This may not be true for the improved GC phases available nowadays.
We have a web page dealing with the occurrence and biochemistry of natural hydroxy fatty acids
here...
We have
a few mass spectra only of 3-pyridylcarbinol ('picolinyl') esters of hydroxy fatty acids available (none of eicosanoids),
and then mainly from standards or well-defined natural sources, so this is a very limited and imperfect review of the topic.
The additional polarity and molecular weight tended to render 3-pyridylcarbinol esters less suitable
for the analysis of complex mixtures (our research interest at one time) by gas chromatography-mass spectrometry.
This may not be true for the improved GC phases available nowadays.
We have a web page dealing with the occurrence and biochemistry of natural hydroxy fatty acids
here...
Mass spectra of methyl esters of hydroxy acids (in underivatized form or as trimethylsilyl ethers), and DMOX/pyrrolidide derivatives are described in separate documents on this website, but there are too many gaps in my accounts to discuss which is best in any systematic way for this specific purpose. The general features of spectra of 3‑pyridylcarbinol esters of fatty acids are discussed in the web page dealing with saturated derivatives.
We were able to obtain very poor yields only of the 3-pyridylcarbinol esters of 2-hydroxy acids for reasons that are not clear, and 3‑hydroxy derivatives proved even more intractable. At the time, we were making our attempts, the transesterification procedure of Destaillats and Angers (2002) had not been published (see the web page on derivative preparation), and this would certainly be worth trying now. Incidentally, we could not prepare dimethyloxazoline derivatives of these isomers either (with one exception), but preparation of pyrrolidides presented no problems.
The mass spectrum of 3-pyridylcarbinyl 2-hydroxy-octadecanoate is -
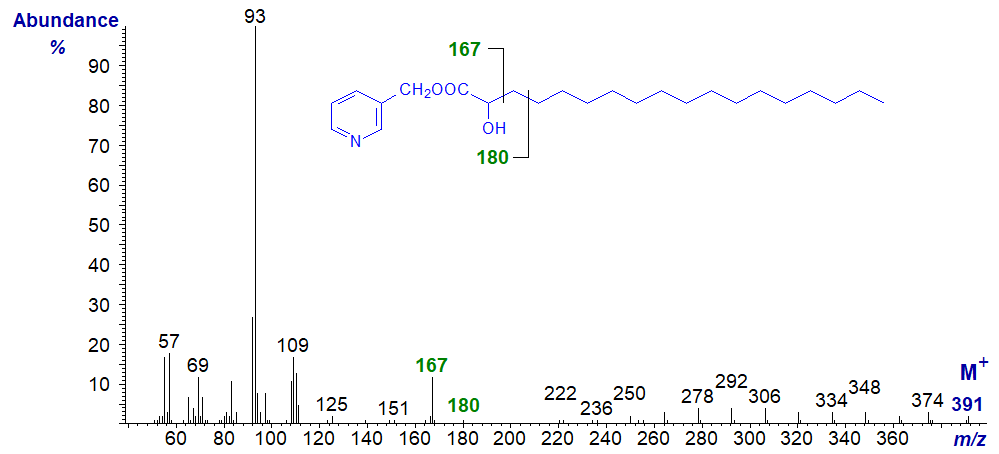
The relative proportions of ions in the high mass region are low. Following the molecular ion, there is a gap of 17 amu for loss of a hydroxyl group, but successive ions thereafter are 14 amu apart for cleavage at each methylene group. The ion for fragmentation between carbons 2 and 3 has moved from m/z = 151 to 167 and must contain the oxygen on carbon 2, while the usual ion expected at m/z = 164 has moved to m/z = 180 (both lower in intensity than usual). The base ion at m/z = 93 is presumed to consist of a protonated pyridine ring with its attached methylene group, but we have no explanation for its relatively high abundance in this instance. Indeed, this is the only spectrum where we have seen this phenomenon.
The mass spectrum of 3-pyridylcarbinyl 12-hydroxystearate (prepared by hydrogenation of ricinoleate) is -
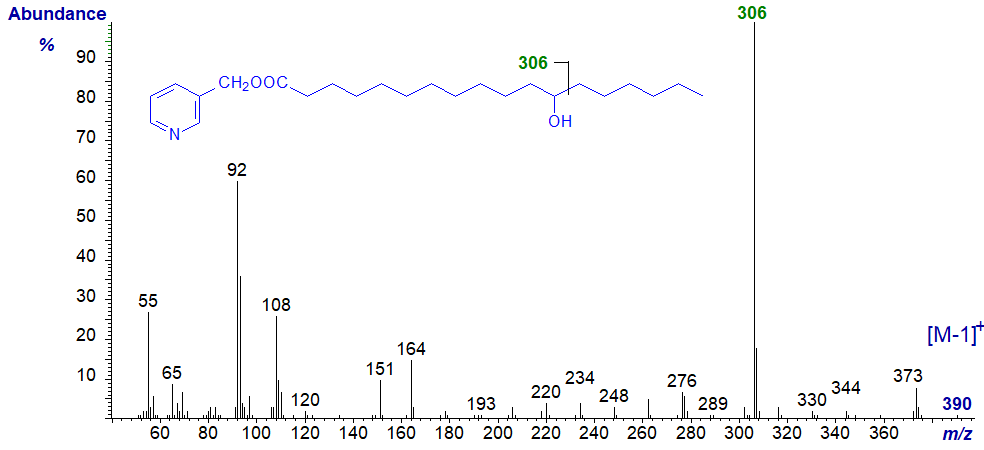
The molecular ion per se is not seen, although there is a small ion representing [M‑1]+, and the first significant ion at m/z = 373 represents the loss of the elements of water. The position of the hydroxyl group is easily confirmed by the base ion at m/z = 306, which is for cleavage alpha to the carbon carrying this group, i.e., between carbons 12 and 13, as illustrated. Spectra of the trimethylsilyl ether derivative of this and of 16‑hydroxy palmitate have been published by Harvey (1984).
The mass spectrum of 3-pyridylcarbinyl ricinoleate (D‑(-)12‑hydroxy-octadec-cis-9-enoate) is equally informative, and the various fragmentations can be interpreted in a straightforward manner to confirm the position of both the hydroxyl group and double bond.
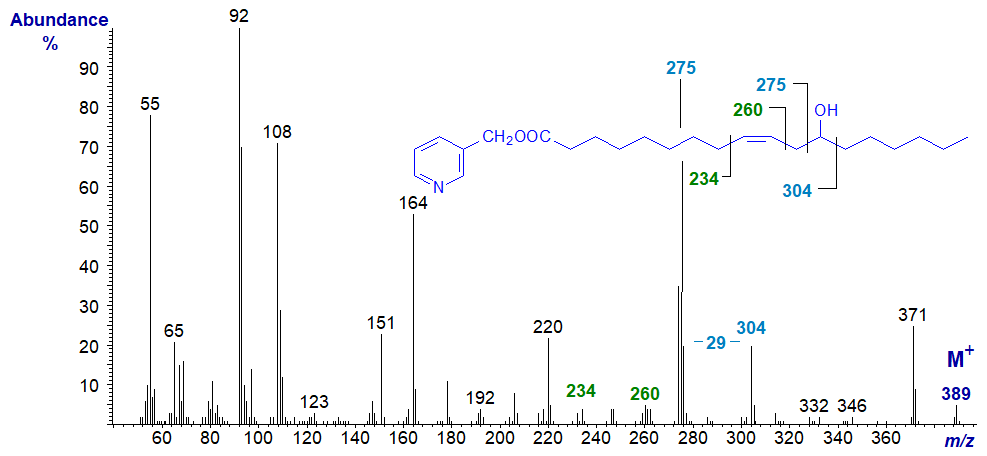
Ions at m/z = 275 (presumably carrying an extra proton) and 304 are produced by cleavage on both sides of the carbon carrying the hydroxyl group to leave a gap of 29 amu as illustrated, in contrast to the pattern with saturated hydroxy acids where the first ion is much less obvious. The double bond in position 9 is most easily recognized by the gap of 40 amu between m/z = 220 and 260 (see our web page on 3 pyridylcarbinol esters of 'monoenoic fatty acids' for an explanation). It must have an influence on the fragmentation at the hydroxyl-carbon to enhance the size of the ion at m/z = 275. Incidentally, we have prepared the trimethylsilyl ether derivative of this, but the spectrum is not helpful for characterization purposes.
The mass spectrum of 3-Pyridylcarbinyl 15-hydroxy-linoleate (a fatty acid found in oat lipids) -
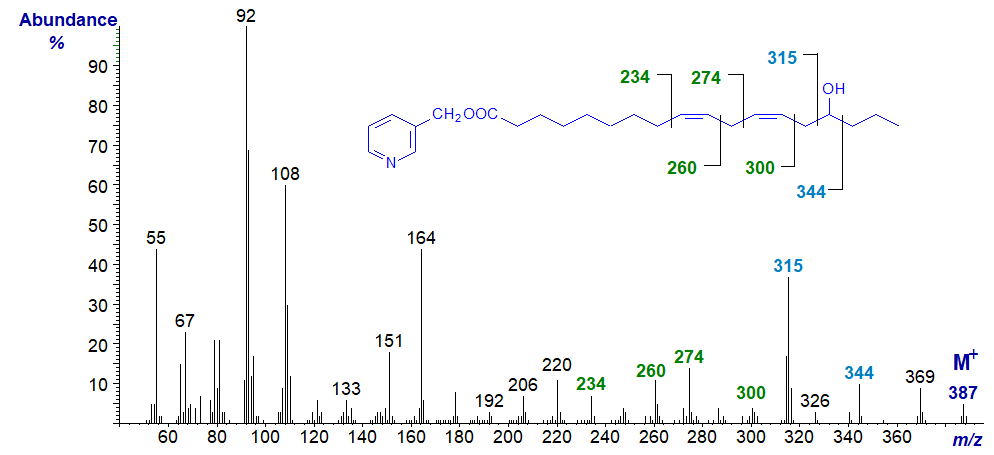
As with the last, there is a distinct molecular ion (m/z = 387). Ions at m/z = 315 and 344 are produced by cleavage on either side of the carbon linked to the hydroxyl group, while the gaps of 26 amu between m/z = 234 and 260 and between 274 and 300 serve to locate the double bonds in positions 9 and 12, respectively (see the web pages on 3-pyridylcarbinol esters of 'dienoic fatty acids' for an explanation).
References
- Destaillats, F. and Angers, P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc., 79, 253-256 (2002); DOI.
- Harvey, D.J. Picolinyl derivatives for the structural determination of fatty acids by mass spectrometry. Applications to polyenoic acids, hydroxy acids, di-acids and related compounds. Biomed. Mass Spectrom., 11, 340-347 (1984); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
