Preparation of Nitrogen-Containing Derivatives
for Mass Spectrometry of Fatty Acids
Methodology is described here for the preparation of those nitrogen-containing derivatives of fatty acids, i.e., 3‑pyridylcarbinol ('picolinyl') esters, 4,4‑dimethyloxazoline (DMOX) derivatives and pyrrolidides, which are most useful for mass spectrometric analysis. I describe laboratory protocols for methods for which I have personal experience only but give brief details of newer procedures that may now have advantages (it is many years since I was last at a work bench). Preparation of methyl ester derivatives is described on a separate web page. Other useful derivatization techniques, e.g., hydrogenation, deuteration, etc., are described in our web page dealing with Mass spectra of methyl esters - further derivatization. I must also recommend my book [1].
Please note! Many of the procedures described below require the use of solvents and other potentially hazardous chemicals, and analysts must take appropriate precautions. It is always sensible to use fume hoods. Troublesome artefacts may be introduced into samples if plastic tubes or pipettes are used at any stage - always use glassware.
1. Hydrolysis
For some derivatives, it can be better to prepare the free (unesterified) acids first by hydrolysis of the lipids with dilute aqueous ethanolic alkali as described here before proceeding to further reactions [1].
| Laboratory protocol: A solution of 0.1M potassium hydroxide in 90% aqueous ethanol is prepared immediately before use by adding 1 volume of standard 1M aqueous potassium hydroxide to 9 volumes of ethanol. To the lipid sample (up to 6 mg) in a stoppered centrifuge tube is added 0.1M potassium hydroxide in 90% aqueous ethanol (0.25 mL per mg sample). Leave at 50°C for 3 hours, then cool and add 2M hydrochloric acid (0.05 mL per mg sample). Add isohexane (3 mL), diethyl ether (3 mL) and distilled water (2 mL). Shake thoroughly and, if necessary (e.g., if there are not two distinct layers and/or an emulsion is formed), centrifuge for 5 min (about 400 rpm). Run the upper organic layer through a short (3 cm) column of anhydrous sodium sulfate to dry it (prepared in a disposable glass Pasteur pipette, plugged with a small piece of cotton wool and pre-washed with 3 mL isohexane-diethyl ether (1:1, by vol.) before use. Collect in a test tube. Repeat the extraction of the lower aqueous layer with isohexane-diethyl ether (3 mL, 1:1, by vol.) and again put the organic layer through the sodium sulfate column. Wash any remaining sample through the column into the tube with more isohexane-diethyl ether (3 mL), before removing the solvent in a gentle stream of nitrogen on a heating block at 30°C. |
2. 3-Pyridylcarbinol ('picolinyl') Esters
3-Pyridylcarbinol esters, commonly but incorrectly known as ‘picolinyl’ esters, are most easily prepared in good yield from the free fatty acids prepared as above followed by activation by conversion to the acid chlorides. Reaction with thionyl chloride is rapid and efficient [2], but the milder method of reaction with oxalyl chloride overnight is also suitable [3]. While there are two steps, it is a single pot reaction. Dry solvents and fresh reagents are required because the reactions are sensitive to moisture.
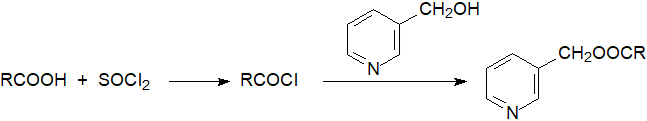 |
| Figure 1. Preparation of 3-pyridylcarbinol esters via acid chlorides. |
| Laboratory protocol: The reactions are carried out in 0.3 ml screw cap vials. The acid (20 µg) is heated with thionyl chloride (20 µL) for 10 min. at 100°C. Excess reagent is evaporated in a stream of nitrogen, a solution of 20% 3-(hydroxy-methyl)pyridine in acetonitrile (10 µL) is added, and the mixture heated for 1 min. at 100°C. An aliquot can be injected directly onto a non-polar GC column, or a clean-up washing procedure as described in the next protocol may be better if a more polar GC phase is in use. |
A mild quantitative method developed for derivatizing sensitive polyunsaturated fatty acids containing epoxyl groups involving an imidazolide intermediate is a useful alternative [4]. It is simple and rapid, involving brief reactions with first carbonyldiimidazole then 3‑(hydroxymethyl)pyridine and a catalyst.
More recently, it was demonstrated that it is possible to prepare 3-pyridylcarbinol esters directly from intact lipids or methyl esters by transesterification with 3-(hydroxymethyl)pyridine in tetrahydrofuran with potassium tert-butoxide as catalyst [5], and a video demonstration of the methodology is available [6]. This reaction may indeed be useful for the preparation of a variety of different ester derivatives by substituting the appropriate alcohol. The reagent must be very dry, otherwise an irreversible hydrolysis reaction can occur. From my limited experience of the reaction, it is best to make a fresh solution of potassium tert-butoxide in dry tetrahydrofuran each time it is required.
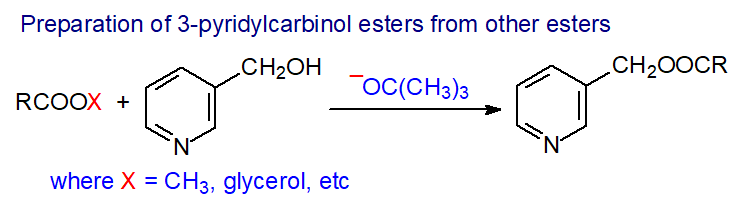 |
| Figure 2. Preparation of 3-pyridylcarbinol esters by base-catalysed transesterification. |
| Laboratory protocol: A solution of potassium tert-butoxide in tetrahydrofuran (0.1 mL, 1.0 M) is added to 3-pyridylcarbinol (0.2 mL). After mixing, the lipid sample (up to 10 mg) in dry dichloromethane (1 mL) is added, and the solution is held at 40°C for 30 min in a closed vial. After cooling to room temperature, water (2 mL) and hexane (4 mL) are added, and the organic phase is collected, dried over anhydrous sodium sulfate and evaporated. The sample is dissolved in isohexane for GC-MS analysis. |
3-Pyridylcarbinol esters can be purified if required by elution from a small column of FlorisilTM with isohexane-diethyl ether (1:4 v/v). They can be converted back to methyl esters by conventional acidic or basic transesterification procedures (see the web page on Preparation of methyl esters) Two further methods for preparation of 3-pyridylcarbinol derivatives have been described recently that look promising, but I have had no opportunity to test them (Sieben, D. et al. Tetrahedron Letts., 57, 808-810 (2016); DOI. Kim, C.S. et al. J. Am. Oil Chem. Soc., 93, 339-346 (2016); DOI).
3. Dimethyloxazoline (DMOX) Derivatives
DMOX derivatives are prepared in a simple one-pot reaction by reaction of lipids with 2-amino-2-methyl-1-propanol in a nitrogen atmosphere at 180°C (a minimum of 2 hours for free acids, or up to 18 hours for methyl esters and intact lipids) [7]. A video demonstration of the methodology is available [6].
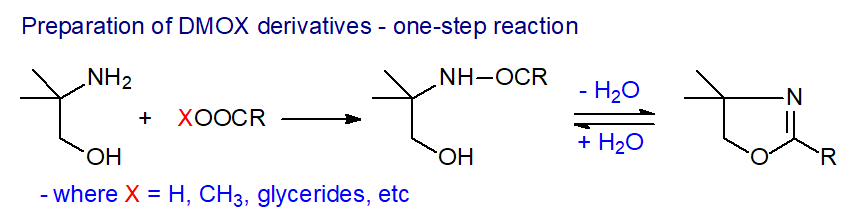 |
| Figure 3. Preparation of DMOX derivatives by the one-pot method. |
| Laboratory protocol: To the lipid sample (up to 2 mg) in a test tube is added 2-amino-2-methyl-1-propanol (0.25 g). The vessel is flushed with nitrogen, stoppered and placed in a heating block at 180°C overnight (or 6 hours for free acids). Flush the tube with nitrogen when the tube is up to temperature to help to eliminate moisture and minimize autoxidation. A little glycerol added to the well of the heating block ensures good contact and that the correct temperature is maintained. Next day, remove the test tube from the heating block, allow to cool to room temperature and wash off any glycerol on the external surface with water. Add diethyl ether-isohexane (1:1, v/v; 5 mL) to the tube, washing down the internal surface, followed by water (5 mL). Shake thoroughly and allow layers to settle. If necessary, addition of a little sodium or potassium chloride will usually break up any interfacial layers. Transfer the organic layer with a Pasteur pipette to a fresh test-tube and re-extract the aqueous layer with fresh solvent (2 mL). Add distilled water (3 mL) to the combined solvent layers, shake and allow to settle. Transfer the solvent layer to a fresh tube with a Pasteur pipette and add anhydrous sodium sulfate (about 1 cm). Leave for one hour with occasional vigorous shaking, then pass the solvent layer through a short (3 cm) column of anhydrous sodium sulfate (prepared in a Pasteur pipette, plugged with a small piece of cotton wool and pre-washed with ca. 3 mL isohexane prior to use) to dry, into a fresh test tube. Wash sample from the sodium sulfate tube through column with isohexane (2 mL), before taking the sample to dryness in a gentle stream of nitrogen on a heating block at 30°C. Dissolve the sample in an appropriate amount of isohexane for GC-MS analysis. Addition of a few crystals of anhydrous sodium sulfate prevents partial hydrolysis of the derivative during storage. |
Unfortunately, we have occasionally observed incomplete reaction and the appearance of the uncyclized intermediate, which elutes later from GC columns (and gives a mass spectrum almost identical to that of the required derivative). It is important to keep the product and reaction mixtures as dry as possible, otherwise traces of moisture can cause ring opening, even on storage in inert solvents at low temperatures [1]. For the same reason, it is not possible to purify DMOX derivatives by adsorption chromatography.
The prolonged high temperature required for the preparation of DMOX derivatives gives cause for concern, and there must be some risk to polyunsaturated fatty acids or any other compound with a labile functional group. For example, trans-3-hexadecenoic acid, common in plant photosynthetic tissue, was found to have isomerized largely to cis-2-hexadecenoic acid during the reaction [8], and crepenynic acid was found to undergo a cyclization reaction [9]. When 2H35-stearic acid was derivatized by this method, the deuterium atoms on carbon 2 were replaced by hydrogen [10], and we have also observed that polyunsaturated acids can be partially isomerized to conjugated isomers if the reaction is carried out carelessly. An alternative two-step reaction has been described that may be safer with such fatty acids. It involves preparing the simple amide via the acid chloride as a first step followed by cyclization with trifluoroacetic anhydride [9]. As with 3‑pyridylcarbinol esters, I prefer to prepare DMOX derivatives via the free acids rather than from intact lipids as the reaction time can be reduced but this is not always practical or convenient.
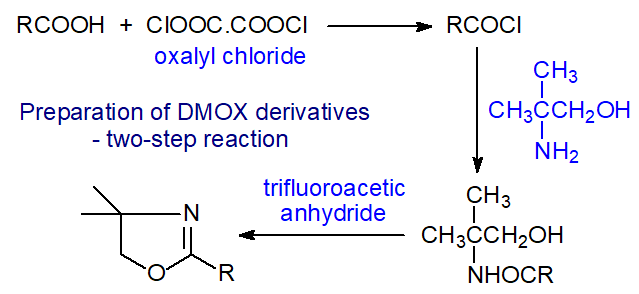 |
| Figure 4. Preparation of DMOX derivatives by a mild two-stage method. |
| Laboratory protocol: The fatty acid must first be converted to the acid chloride as in the methods for preparation of 3-pyridylcarbinol esters via the acid chloride. This is used immediately for the preparation of the required derivatives. A solution of 2-amino-2-methylpropanol in dichloromethane (10 mg/mL) is prepared and stored over anhydrous sodium sulfate. It is stable in the refrigerator for about 1 month. This solution (0.5 mL) is added to the freshly prepared acid chloride, cooled in an ice bath, and the mixture is then left to warm up to room temperature for an hour. The solvent is removed in a stream of nitrogen, and trifluoroacetic anhydride (0.5 mL) is added. After 1 hour on a heating block at 50°C, or 3 hours at room temperature, the reagent is removed in a stream of nitrogen. Isohexane (5 mL) is added followed by water (2 mL), and the product is obtained as in the previous reaction, taking care to dry the product thoroughly. |
A new mild procedure for preparing DMOX derivatives and pyrrolidides from free acids using Deoxo-FluorTM reagent to activate the fatty acids, which should be applicable to pyridylcarbinol esters, has been described [11], but a better option may be a reaction is facilitated by the presence of sodium borohydride, as it can be carried out on methyl esters at room temperature [12]. We have yet to try either of these methods so would be interested in learning of the experience of others. Of the many alternative nitrogen-containing derivatives for mass spectrometry that have been described in the literature, simple oxazolines appear to me to be the only ones that might be worth pursuing [13], although few published spectra are available for comparison purposes.
When necessary, DMOX derivatives can be converted back to methyl esters by the methylation procedures used for sphingolipids, e.g., by reaction with methanol containing hydrochloric acid [1].
4. Pyrrolidides
Pyrrolidides are prepared simply by reacting methyl esters of fatty acids with pyrrolidine in the presence of acetic acid [14].
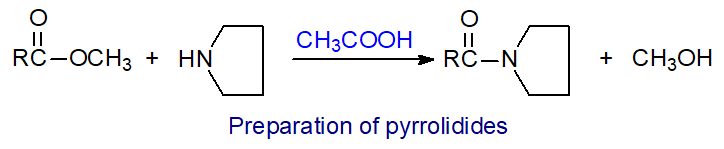 |
| Figure 5. Preparation of pyrrolidides. |
| Laboratory protocol: The fatty acid methyl ester (up to 10 mg) is dissolved in freshly distilled pyrrolidine (1 mL), acetic acid (0.1 mL) is added, and the mixture is heated at 100°C for 1 hour. Excess pyrrolidine is blown off in a stream of nitrogen at 50°C, and then the residue is taken up in hexane-diethyl ether (1:1, v/v; 8 mL) and is washed three times with water (4 mL portions). After drying over anhydrous sodium sulfate, the required product is obtained on evaporation of the solvent. |
The reaction takes place under basic conditions and is relatively mild, so it may be safer with some fatty acids containing labile functional groups than preparation of DMOX derivatives. Although I have no personal experience, a better option may be a new mild procedure facilitated by the presence of sodium borohydride [12]. Alternative methods are available to prepare pyrrolidides directly from triacylglycerols and presumably other glycerolipids [15] or from free fatty acids [11], the latter in a rapid one-pot reaction.
References
 Christie, W.W.
and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis
(4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see
Science Direct.
Christie, W.W.
and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis
(4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see
Science Direct.- Harvey, D.J. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom., 9, 33-38 (1982); DOI.
- Mattson, F.H. and Volpenhein, R.A. Synthesis and properties of glycerides. J. Lipid Res., 3, 281-296 (1962); DOI.
- Balazy, M. and Nies, A.S. Characterization of epoxides of polyunsaturated fatty acids by mass spectrometry via 3‑pyridinylmethyl esters. Biomed. Environ. Mass Spectrom., 18, 328-336 (1989); DOI.
- Destaillats, F. and Angers, P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc., 79, 253-256 (2002); DOI.
- Ginies, C., Brillard, J. and Nguyen-The, C. Identification of fatty acids in Bacillus cereus.. J. Vis. Exp., 118, e54960 (2016); DOI.
- Fay, L. and Richli, U. Location of double bonds in polyunsaturated fatty acids by gas chromatography-mass spectrometry after 4,4‑dimethyloxazoline derivatization. J. Chromatogr. A, 541, 89-98 (1991); DOI.
- Lamberto, M. and Ackman, R.G. Positional isomerization of trans-3-hexadecenoic acid employing 2-amino-2-methylpropanol as a derivatizing agent for double bond location by GC/MS. Anal. Biochem., 230, 224-228 (1995); DOI.
- Christie, W.W. Mass spectrometry of fatty acids with methylene-interrupted ene-yne systems. Chem. Phys. Lipids, 94, 35-41 (1998); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Kangani, C.O. and Kelley, D.E. One pot direct synthesis of amides or oxazolines from carboxylic acids using Deoxo-Fluor reagent. Tetrahedron Letts., 46, 8917-8920 (2005); DOI.
- Santalova, E.A. and Svetashev, V.I. Preparation of 4,4-dimethyloxazoline and pyrrolidine derivatives from fatty acid methyl esters using sodium borohydride: mild and simple one-pot derivatization procedures for a gas chromatographic-mass spectrometric analysis of fatty acids. Nat. Prod. Res., 17, 1934578X221131408 (2022); DOI.
- Kuklev, D.V. and Smith, W.L. A procedure for preparing oxazolines of highly unsaturated fatty acids to determine double bond positions by mass spectrometry. J. Lipid Res., 44, 1060-1066 (2003); DOI.
- Andersson, B.A. and Holman, R.T. Pyrrolidides for mass spectrometric determination of the position of double bonds in monounsaturated fatty acids. Lipids, 9, 185-190 (1974); DOI.
- Vetter, W. and Walther, W. Pyrrolidides as derivatives for the determination of the fatty acids of triacylglycerols by gas-chromatography. J. Chromatogr. A, 686, 149-154 (1994); DOI.
| © Author: William W. Christie |  |
|
| Updated: January 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
