Mass Spectrometry of 3-Pyridylcarbinol Esters
Penta- and Hexaenoic Fatty Acids
 The
greater complexity of the mass spectra of 3-pyridylcarbinol esters of polyenoic fatty acids with five or six double bonds
can make interpretation even more difficult than with the comparable triene
and tetraene derivatives.
On the other hand, the availability of standards or authentic spectra for comparison purposes does make the task somewhat easier,
as each isomer always has a distinctive fingerprint.
The main problem is usually in locating the first double bond in the chain.
The principles of locating double bonds via 3‑pyridylcarbinol esters are discussed in the web page
on 3‑pyridylcarbinol esters of monoenoic acids and in subsequent documents,
but in brief we are looking for gaps of 26 amu for each double bond or better for gaps of 40 amu
for the double bond and the preceding methylene group.
The main fragments here are illustrated simplistically but are not discussed in any detail.
It is worth noting that these and other nitrogen-containing derivatives always give a distinctive molecular ion
with polyunsaturated fatty acids in contrast to the methyl esters.
The web page on 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature,
but many of the following spectra will not have been published elsewhere.
The
greater complexity of the mass spectra of 3-pyridylcarbinol esters of polyenoic fatty acids with five or six double bonds
can make interpretation even more difficult than with the comparable triene
and tetraene derivatives.
On the other hand, the availability of standards or authentic spectra for comparison purposes does make the task somewhat easier,
as each isomer always has a distinctive fingerprint.
The main problem is usually in locating the first double bond in the chain.
The principles of locating double bonds via 3‑pyridylcarbinol esters are discussed in the web page
on 3‑pyridylcarbinol esters of monoenoic acids and in subsequent documents,
but in brief we are looking for gaps of 26 amu for each double bond or better for gaps of 40 amu
for the double bond and the preceding methylene group.
The main fragments here are illustrated simplistically but are not discussed in any detail.
It is worth noting that these and other nitrogen-containing derivatives always give a distinctive molecular ion
with polyunsaturated fatty acids in contrast to the methyl esters.
The web page on 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature,
but many of the following spectra will not have been published elsewhere.
Pentaenoic Fatty Acids
The spectrum of 3-pyridylcarbinyl 5,8,11,14,17-eicosapentaenoate (20:5(n-3) or 'EPA') is the first to be illustrated (Wolff et al., 1999). This is arguably the most important of the natural pentaenes, as it is an essential constituent of the phospholipids in animal tissues, especially in brain, and it is the precursor of the PG3 series of prostaglandins.
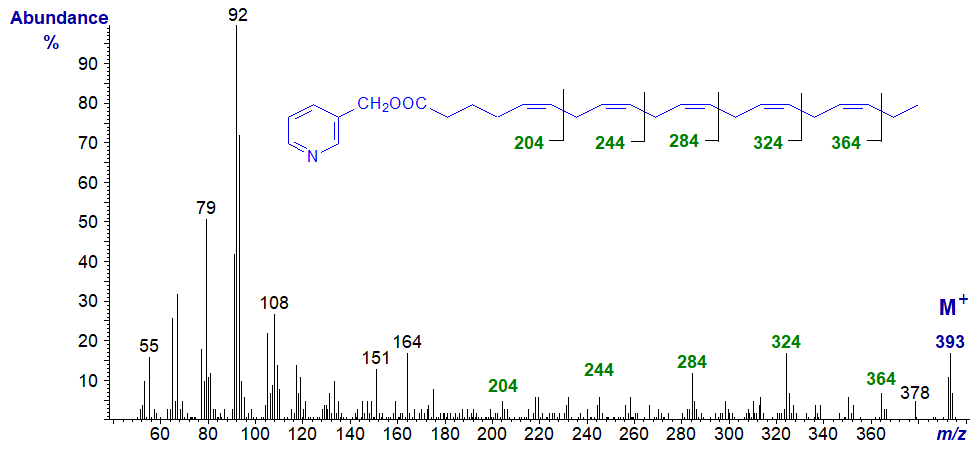
The expected gaps of 26 amu for fragmentations at the double bond are not always easy to see, although they are indeed present, but those of 40 amu for the double bond and an associated methylene group can be distinguished.
3-Pyridylcarbinyl 3,6,9,12,15-octadecapentaenoate (18:5(n-3)) -
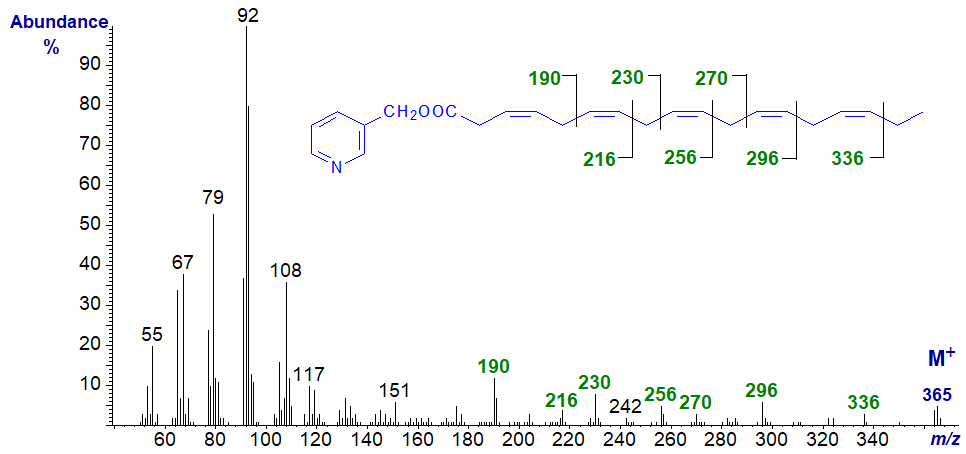
It was quite an achievement to obtain a spectrum of this acid, which is a minor component of some marine organisms, as the double bond in position 3 isomerizes very readily on attempting to prepare derivatives (Prof. M.V. Bell of Stirling University kindly provided a sample). Interpretation is as described elsewhere for tri- and tetraenoic fatty acid derivatives. Note that the usual ions at m/z = 151 and 164 are scarcely apparent. Again, the double bonds are most easily located from the gaps of 40 amu, although the expected gaps of 26 amu are detectable for all but the first double bond. The isomerized product, 2,6,9,12,15-octadecapentaenoate, has a very different spectrum with the base ion at m/z = 177 for cleavage at the centre of the bis-methylene interrupted double bond system (author, unpublished - available in the Archive pages here...).
3-Pyridylcarbinyl 4,7,10,13,16-docosapentaenoate (22:5(n-6)). All but the first double bond are easily located from the ions marked, and the same is true for the following spectrum of the n-3 isomer.
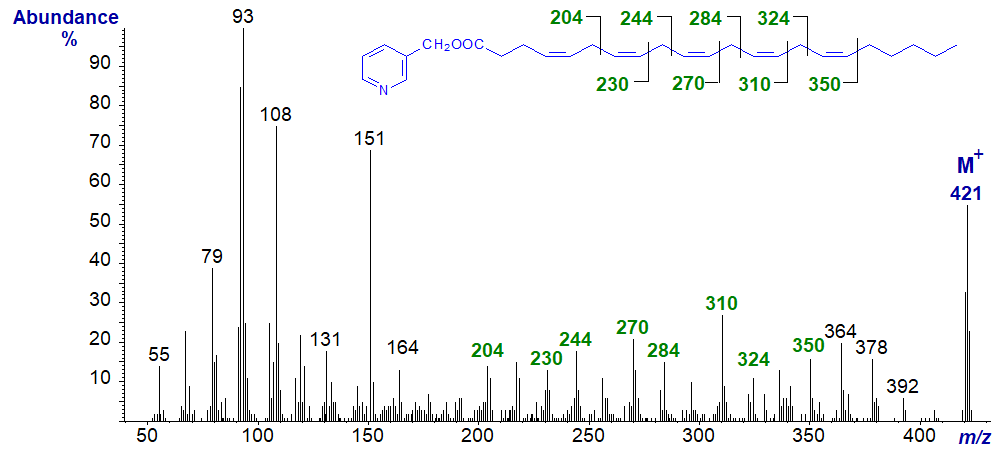
3-Pyridylcarbinyl 7,10,13,16,19-docosapentaenoate (22:5(n-3)) -
The mass spectrum of the 3-pyridylcarbinol derivative of 6,9,12,15,18-heneicosapentaenoate (21:5(n-3)), a minor but ubiquitous component of fish oils, and that of 9,12,15,18,21-tetracosapentaenoate (24:5(n-3)) are illustrated in our Archive section, but without interpretation.
Hexaenoic Fatty Acids
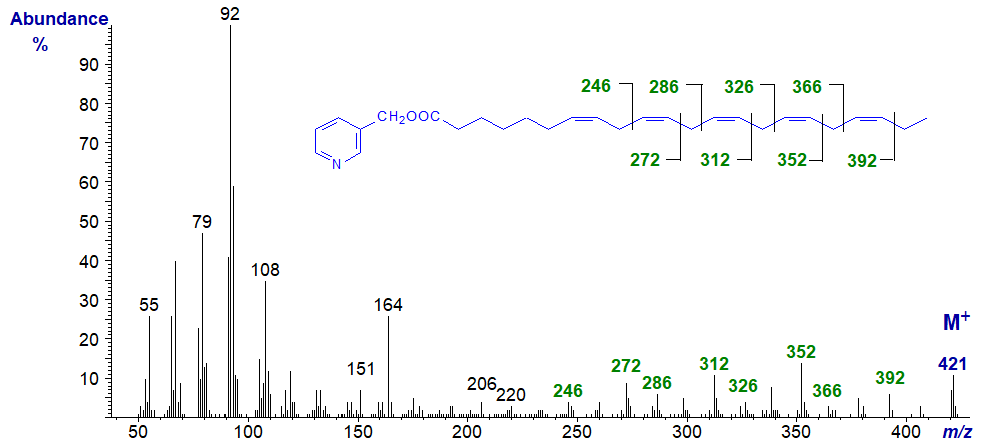
The mass spectrum of 3-pyridylcarbinyl 4,7,10,13,16,19-docosahexaenoate (22:6(n-3) or ‘DHA’) is illustrated (Harvey, 1984). This is a key essential fatty acid of the n-3 family, especially in the brain, and is the precursor of a family of docosanoids, including the protectins and resolvins. The molecular ion is easy to distinguish in comparison to spectra of methyl esters, for example.
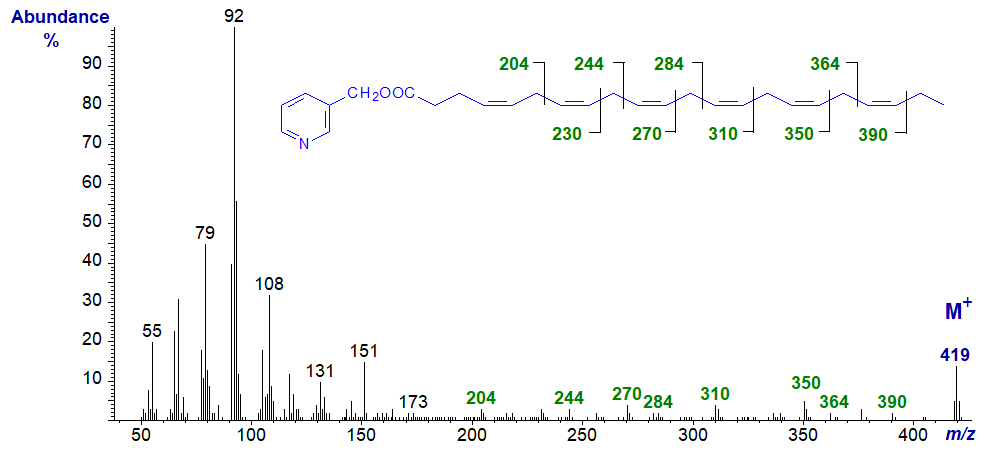
For this and the next spectrum, diagnostic ions can indeed be located for all but the first double bond relative to the carboxyl group as indicated, but overall, they are perhaps best regarded simply as fingerprints.
3-Pyridylcarbinyl 6,9,12,15,18,21-tetracosahexaenoate (24:6(n-3)), the biosynthetic precursor of the previous fatty acid by the Sprecher pathway -
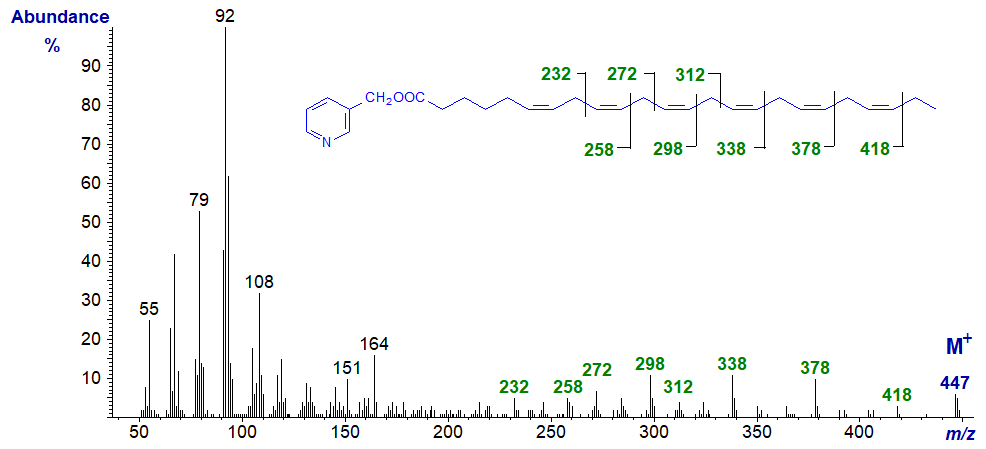
Further relevant spectra are available in our Archive section, but without interpretation.
References
- Harvey, D.J. Picolinyl derivatives for the structural determination of fatty acids by mass spectrometry. Applications to polyenoic acids, hydroxy acids, di-acids and related compounds. Biomed. Mass Spectrom., 11, 340-347 (1984); DOI.
- Wolff, R.L., Christie, W.W., Pedrono, F. and Marpeau, A.M. Arachidonic, eicosapentaenoic, and biosynthetically related fatty acids in the seed lipids from a primitive gymnosperm, Agathis robusta. Lipids, 34, 1083-1097 (1999); DOI.
See also - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
