Mass Spectrometry of 3-Pyridylcarbinol Esters
Tetraenoic Fatty Acids
 As with trienes,
the mass spectra of 3-pyridylcarbinol esters of tetraenoic fatty acids permit location of the double bonds,
but less easily than those of monoenes and dienes.
The diagnostic ions often occur at the centres of clusters and do not stand out well, but different isomers tend to have very different
spectra so that characterization is possible when spectra of authentic fatty acids can be compared.
Few model compounds are available, so most of the following spectra have been obtained from analyses of those natural products encountered
in our research activities, and representatives of fatty acids with a variety of chain-lengths are described.
The web page on the pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature,
but most of the spectra that follow will not have been published formally elsewhere.
As with trienes,
the mass spectra of 3-pyridylcarbinol esters of tetraenoic fatty acids permit location of the double bonds,
but less easily than those of monoenes and dienes.
The diagnostic ions often occur at the centres of clusters and do not stand out well, but different isomers tend to have very different
spectra so that characterization is possible when spectra of authentic fatty acids can be compared.
Few model compounds are available, so most of the following spectra have been obtained from analyses of those natural products encountered
in our research activities, and representatives of fatty acids with a variety of chain-lengths are described.
The web page on the pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature,
but most of the spectra that follow will not have been published formally elsewhere.
Methylene-Interrupted Tetraenoic Fatty Acids
The mass spectrum of 3-pyridylcarbinyl 6,9,12,15-octadecatetraenoate (stearidonate or 18:4(n-3)) is illustrated below (Griffiths et al., 1996; Wolff et al., 1999) -
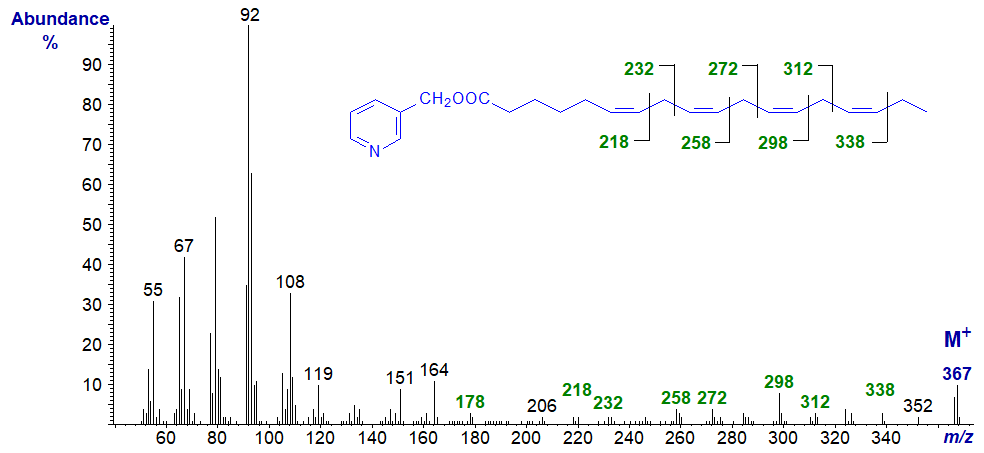
The principles of double bond location are described in our web page on 3-pyridylcarbinol esters of monoenoic fatty acids. The gap of 26 amu between m/z = 312 and 338 locates the terminal double bond rather easily, and the gap between m/z = 272 and 298 locates that in position 12, but the first double bonds are not so readily identified. However, if the first double bond were in a different position, the spectrum would change (see that for 3‑pyridylcarbinyl 5,9,12,15-octadecatetraenoate below, for example). As with trienes, it is often easier to spot gaps of 40 amu for the double bond and an associated methylene group, in this instance from m/z = 178 to 218 to 258 to 298 to 338. The same principles apply to interpretation of mass spectra of structurally related compounds.
3-Pyridylcarbinyl 8,11,14,17-octadecatetraenoate (18:4(n-1)), from a fatty acid that is occasionally found as a minor component of marine organisms -
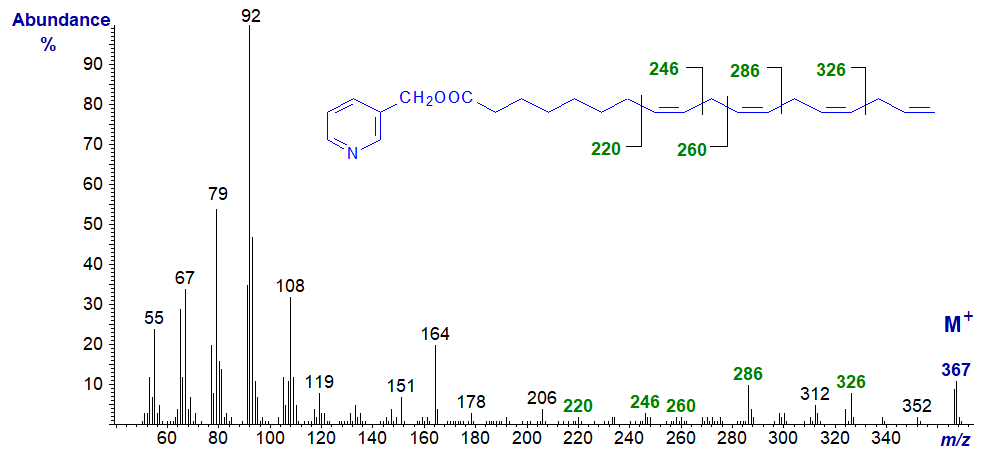
In this instance, the presence of the terminal double bond causes rearrangements that confuse the interpretation (a problem with all derivatives of this type, including DMOX derivatives and pyrrolidides), but the first two double bonds are easily located without dubiety, as indicated on the spectrum.
We have spectra on file for 4,7,10,13-hexadecatrienoate (16:4(n-3)) and 6,9,12,15-hexadecatrienoate (16:4(n-1)) on file, but without interpretation in our Archive section.
Arachidonic acid is a key essential fatty acid in animal tissues and the precursor of families of eicosanoids or prostaglandins, and the spectrum of 3‑pyridylcarbinyl 5,8,11,14-eicosatetraenoate (20:4(n-6)) follows (Harvey, 1984; Wolff et al., 1999).
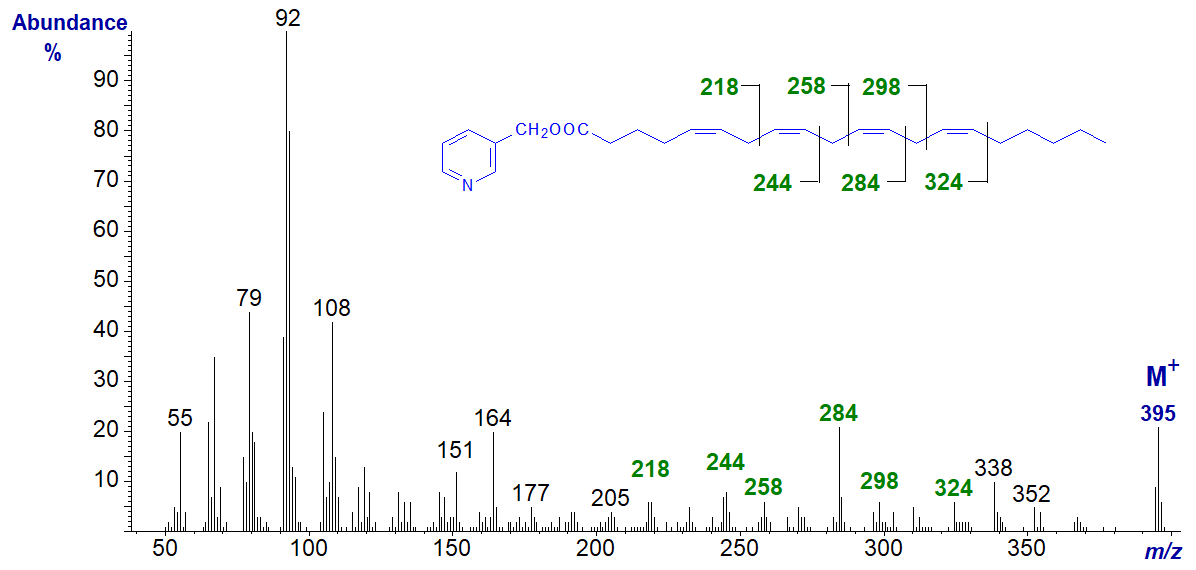
The first double bond is not easily located, but the remaining three are from the fragment ions that are annotated. The same diagnostic ions are present in the spectrum of 5,8,11,14-19:4.
Similar reasoning applies to the spectrum of 3-pyridylcarbinyl 8,11,14,17-eicosatetraenoate (20:4(n-3)) -
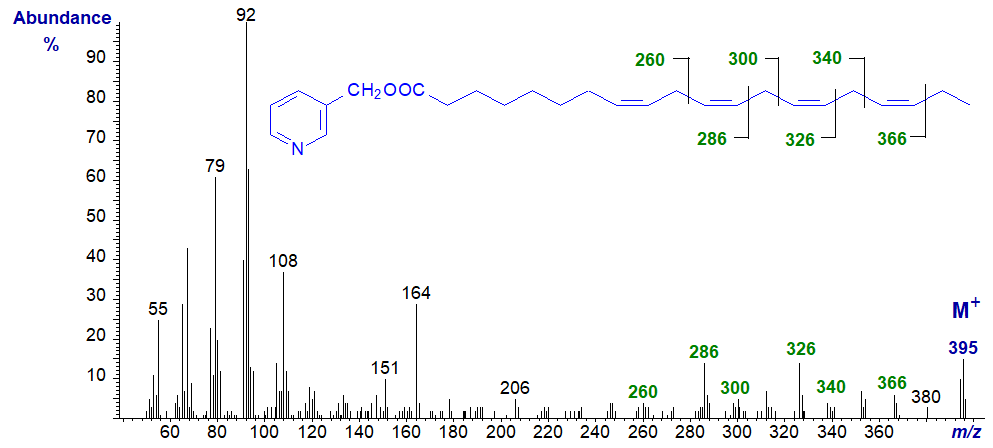
On the other hand, when the double bonds are farther from the carboxyl group, as with 3-pyridylcarbinyl 9,12,15,18-tetracosanoate (24:4(n‑6)), they are all relatively easy to locate.
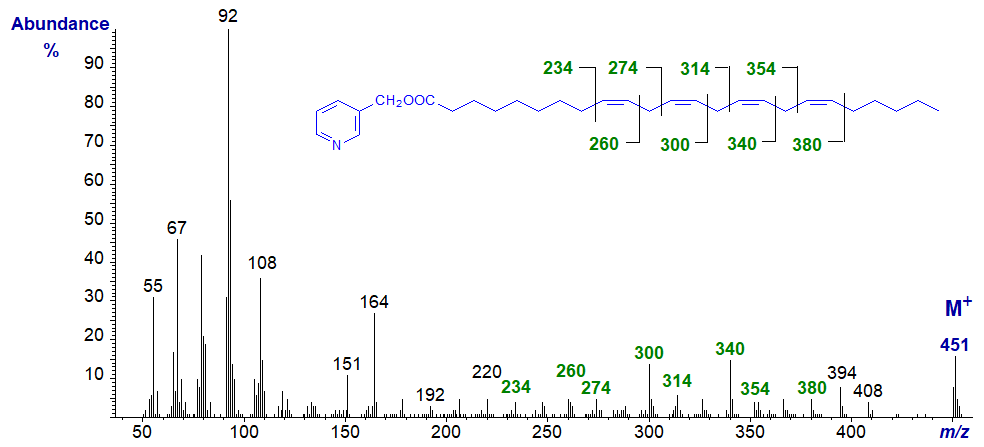
Further relevant spectra are available in our Archive section (but without interpretation).
Bis- and Poly-Methylene-Interrupted Tetraenoic Fatty Acids
As described elsewhere for trienes, it has become apparent that bis- and polymethylene-interrupted trienoic fatty acids are more common in nature than may have been supposed, and fatty acids with a 5,9-double bond system or their chain elongation products are common in seed oils from Gymnosperms (conifers) and in certain marine invertebrates such as sponges. The spectrum of 3-pyridylcarbinyl 5,9,12,15-octadecatetraenoate from a Gymnosperm is typical -
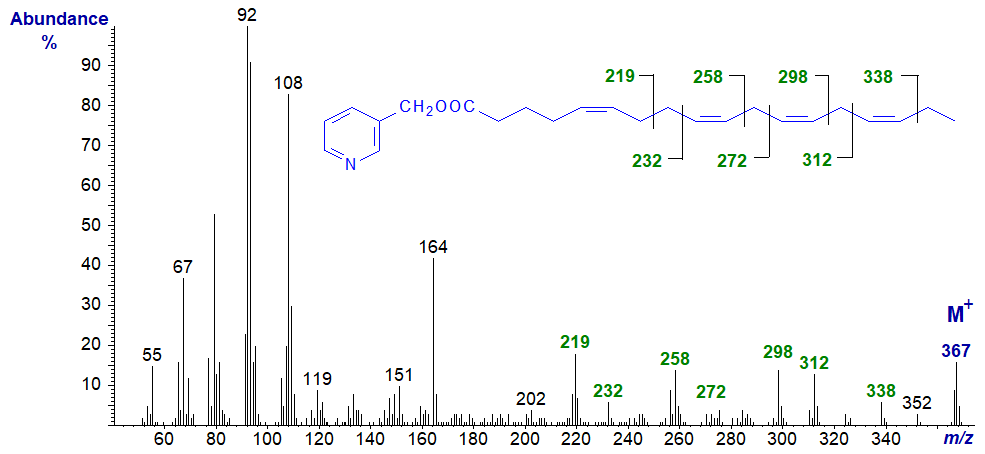
The prominent ion at m/z = 219 represents cleavage between carbons 7 and 8, i.e., the centre of the bis-methylene-interrupted double bond system, as described first in the web pages on 3-pyridylcarbinol esters of dienes - part 2. The remaining double bonds are easily located from the ions that are annotated. It may be helpful to compare it with that of 5,9,12-18:3 in the web pages dealing with 3‑pyridylcarbinol esters of trienes).
3-Pyridylcarbinyl 5,11,14,17-eicosatetraenoate (also from a Gymnosperm) (Wolff et al., 1999) -
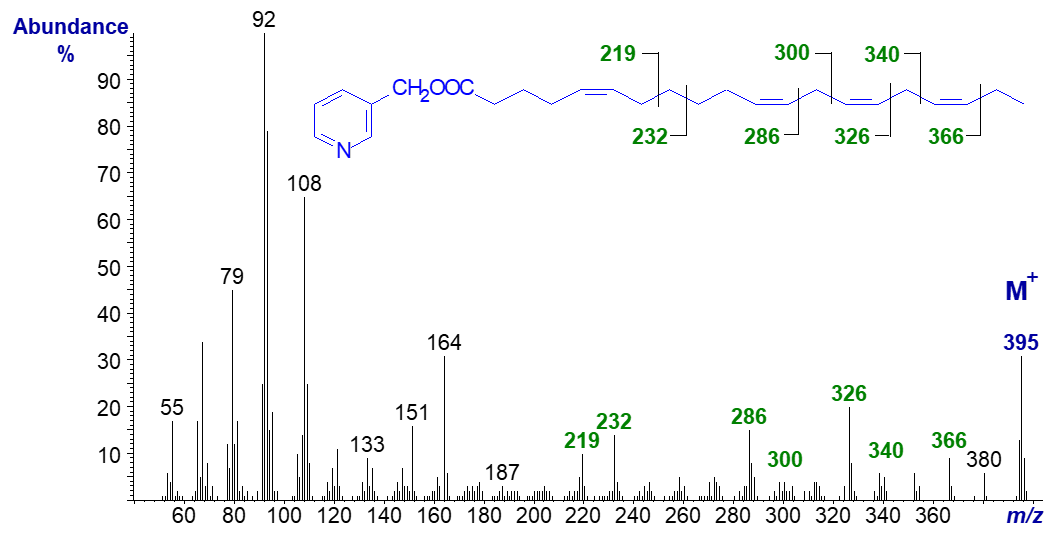
Again, distinctive ions serve to locate the double bonds, and the spectrum can be compared with that of the analogous triene, 3‑pyridylcarbinyl 5,11,14‑eicosatrienoate (Mass spectra of 3-pyridylcarbinol esters - trienoic fatty acids).
Definitive spectra have been obtained from some very-long-chain fatty acid derivatives, such as 3‑pyridylcarbinyl 5,9,19,23-triacontatetraenoate (5,9,19,23‑30:4) from a sponge Joh et al., 1997).
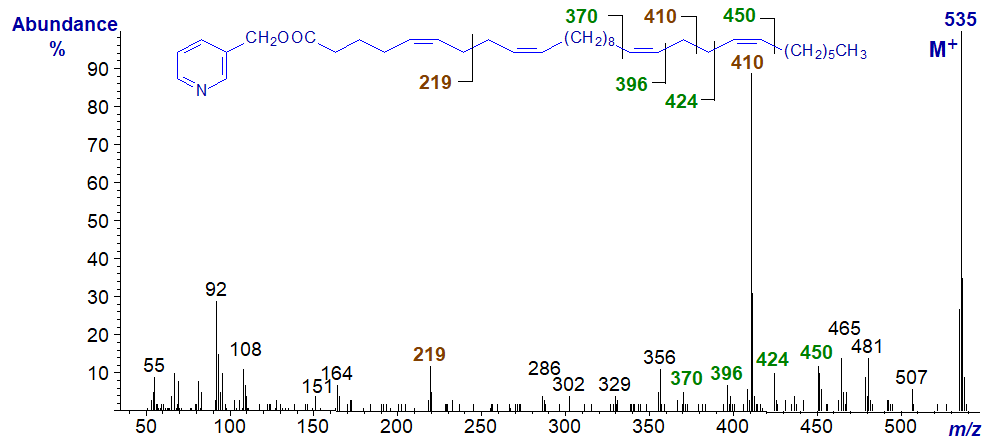
This has two bis-methylene-interrupted double bond systems, and there is little doubt that they are defined by the ions at m/z = 219 and 410, while further useful ions serve to confirm the positions of the last two double bonds at least.
Further relevant spectra are available in our Archive section (but without interpretation).
References
- Griffiths, G., Brechany, E.Y., Jackson, F.M., Christie, W.W., Stymne, S. and Stobart, A.K. Distribution and biosynthesis of stearidonic acid in leaves of Borago officinalis. Phytochemistry, 43, 381-386 (1996); DOI.
- Harvey, D.J. Picolinyl derivatives for the structural determination of fatty acids by mass spectrometry. Applications to polyenoic acids, hydroxy acids, di-acids and related compounds. Biomed. Mass Spectrom., 11, 340-347 (1984); DOI.
- Joh, Y.G., Elenkov, I.J., Stefanov, K.L., Popov, S.S., Dobson, G. and Christie, W.W. Novel di-, tri-, and tetraenoic fatty acids with bis-methylene-interrupted double-bond systems from the sponge Haliclona cinerea. Lipids, 32, 13-17 (1997); DOI.
- Wolff, R.L., Christie, W.W., Pedrono, F. and Marpeau, A.M. Arachidonic, eicosapentaenoic, and biosynthetically related fatty acids in the seed lipids from a primitive gymnosperm, Agathis robusta. Lipids, 34, 1083-1097 (1999); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
