Mass Spectrometry of 3-Pyridylcarbinol Esters
Trienoic Fatty Acids
 The
mass spectra of 3-pyridylcarbinol esters of trienoic fatty acids permit location of the double bonds,
but less easily than those of monoenes and dienes.
The diagnostic ions often occur at the centres of clusters and do not stand out as well,
especially for the double bond closest to the carboxyl group.
As different isomers tend to have very different spectra, characterization is possible when comparisons can be made with spectra of
authenticated derivatives or standards.
Unlike the dienes, few model compounds are available, so most of the following spectra have been gleaned from analyses of
those natural products encountered in our research, and fatty acids with a variety of chain-lengths are described.
There are three sections dealing with methylene-interrupted, conjugated, and bis- and polymethylene-interrupted trienes.
The web page on 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature.
The
mass spectra of 3-pyridylcarbinol esters of trienoic fatty acids permit location of the double bonds,
but less easily than those of monoenes and dienes.
The diagnostic ions often occur at the centres of clusters and do not stand out as well,
especially for the double bond closest to the carboxyl group.
As different isomers tend to have very different spectra, characterization is possible when comparisons can be made with spectra of
authenticated derivatives or standards.
Unlike the dienes, few model compounds are available, so most of the following spectra have been gleaned from analyses of
those natural products encountered in our research, and fatty acids with a variety of chain-lengths are described.
There are three sections dealing with methylene-interrupted, conjugated, and bis- and polymethylene-interrupted trienes.
The web page on 3‑pyridylcarbinol esters of saturated fatty acids contains more introductory and
mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration,
derivative preparation, etc.).
References are listed when we are aware of prior formal publication of spectra in the scientific literature.
Methylene-Interrupted Trienoic Fatty Acids
The mass spectrum of 3-pyridylcarbinyl 6,9,12-octadecatrienoate (γ-linolenate or 18:3(n-6)) (from Wolff et al., 1999) is illustrated first -
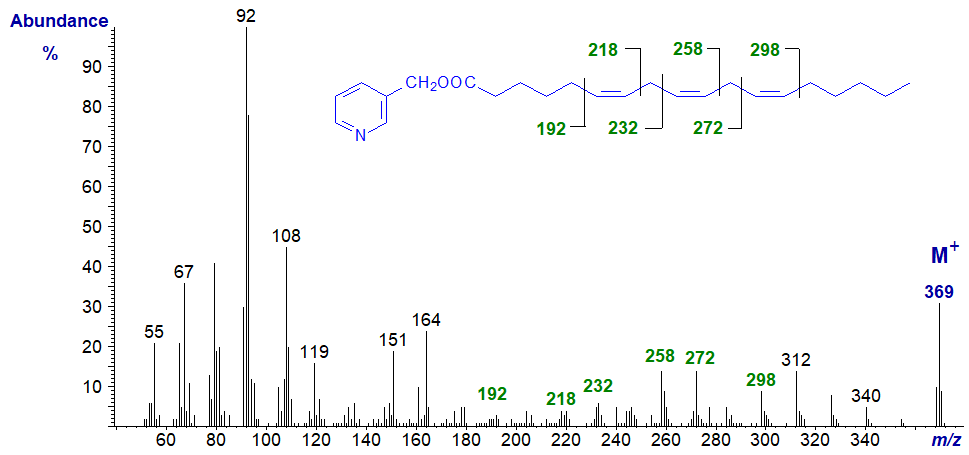
The principles of double bond location are described in our web page on 3-pyridylcarbinol esters of monoenoic fatty acids. In brief, we start with the molecular ion, then look for a break in the regular series of ions 14 amu apart for successive methylene groups. The gap of 26 amu between m/z = 272 and 298 locates the terminal double bond rather easily, and the gap between m/z = 232 and 258 locates that in position 9, but the diagnostic ions for first double bond are not easily seen. If the first double bond were in any other position than 6, the spectrum would be very different (see that for 3-pyridylcarbinyl 5,9,12-octadecatrienoate below, for example). Often a gap of 40 amu for the double bond and the associated methylene group on the carboxyl side of the molecule is easier to spot, as can be seen in the next spectrum.
3-Pyridylcarbinyl 9,12,15-octadecatrienoate (α-linolenate or 18:3(n-3)) -
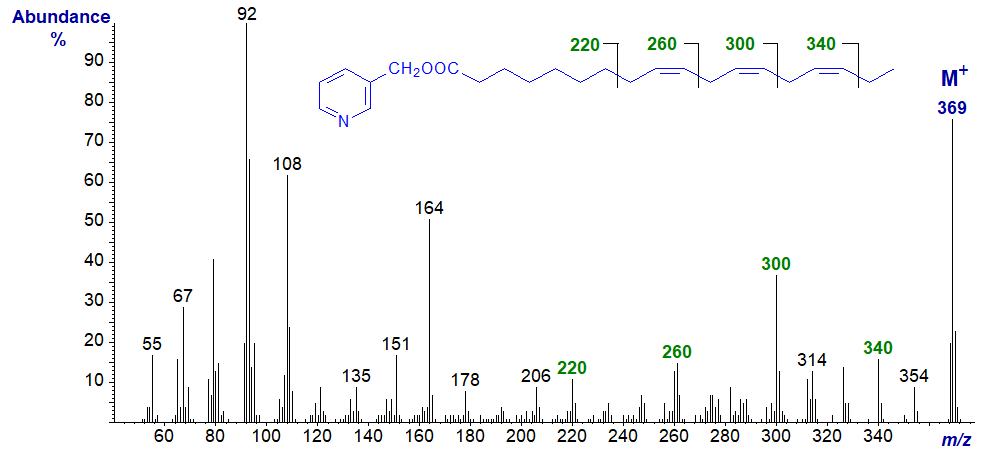
Here the gaps of 40 discussed above are between m/z = 220 and 260, 260 and 300, and 300 and 340, as illustrated (Harvey, 1984). In this instance, the gaps of 26 amu for the double bond are obscure for the first two double bonds. Similar principles apply to interpretation of spectra of structurally related compounds, including the following of which some are important biologically. In most, I have simply emphasized the key ions rather than giving a detailed description.
3-Pyridylcarbinyl 4,7,10-hexadecatrienoate (16:3(n-6)) - diagnostic ions 28 amu less than for 6,9,12-18:3 above.
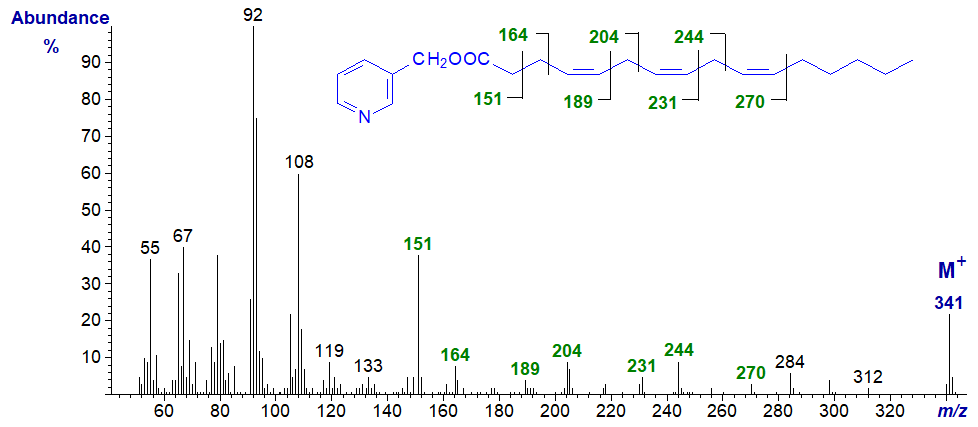
3-Pyridylcarbinyl 6,9,12-hexadecatrienoate (16:3(n-4)) - the diagnostic ions are the same as those for 6,9,12-18:3 above.
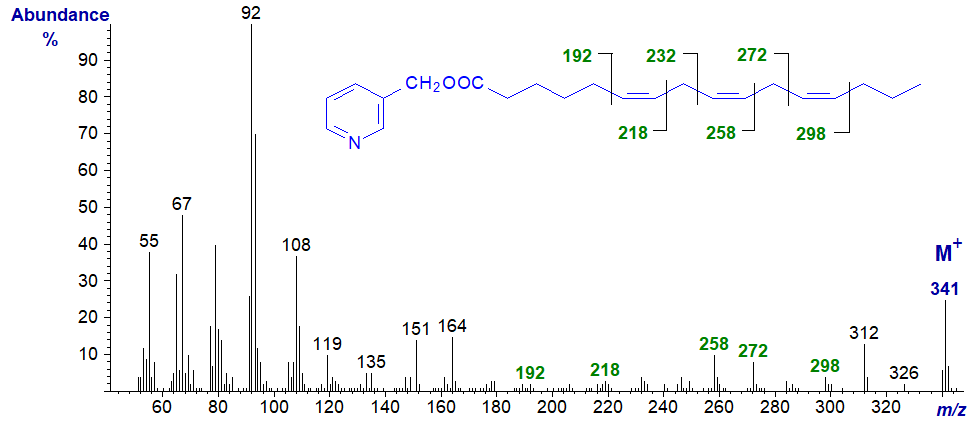
3-Pyridylcarbinyl 7,10,13-hexadecatrienoate (16:3(n-3)). The last two doubles bonds are easily located, but not the first; note that the 'fingerprint' is distinctly different from that of other 16:3 isomers and is sufficient for definitive characterization when a standard spectrum is available for comparison.
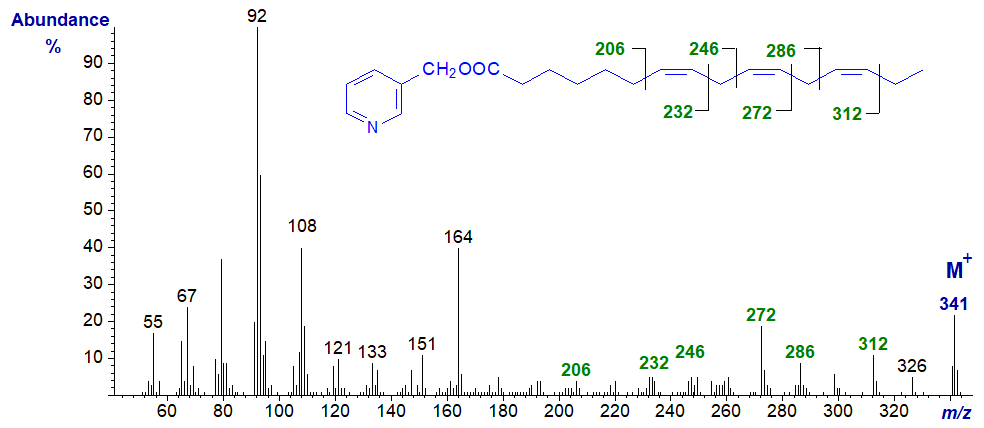
Now the biologically important 20:3 isomers, starting with 3-pyridylcarbinyl 5,8,11-eicosatrienoate (20:3(n-9) - Mead's acid), an acid that accumulates in essential fatty acid deficiency (Christie et al., 1986, Adkisson et al., 1991). Only the last two double bonds are easily located from first principles.
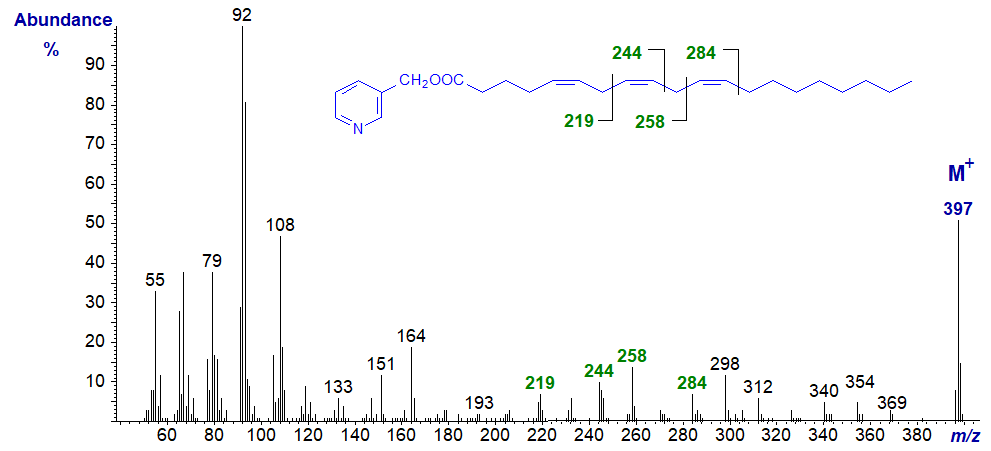
3-Pyridylcarbinyl 8,11,14-eicosatrienoate (20:3(n-6)) (from - Christie et al., 1986) -
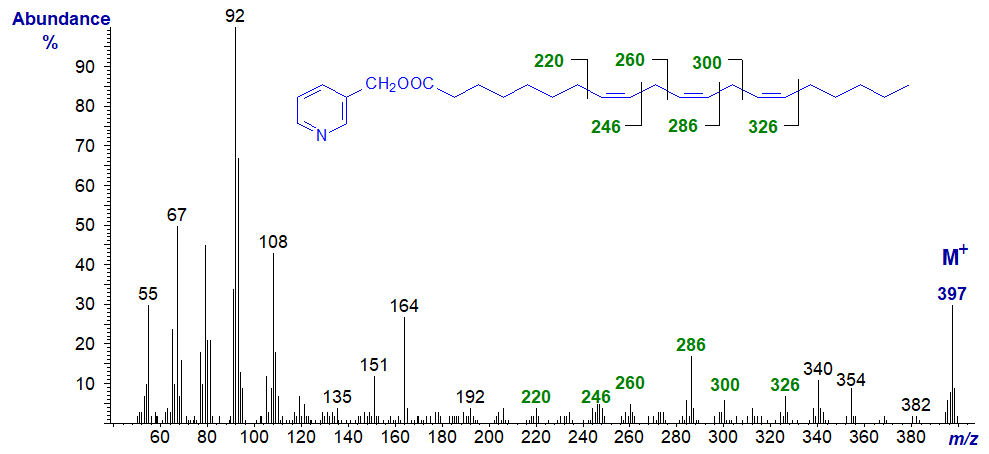
The same diagnostic ions are present in the spectra illustrated elsewhere on this site of the 3-pyridylcarbinol esters of 8,11,14-17:3 and 8,11,14-19:3, but without interpretation.
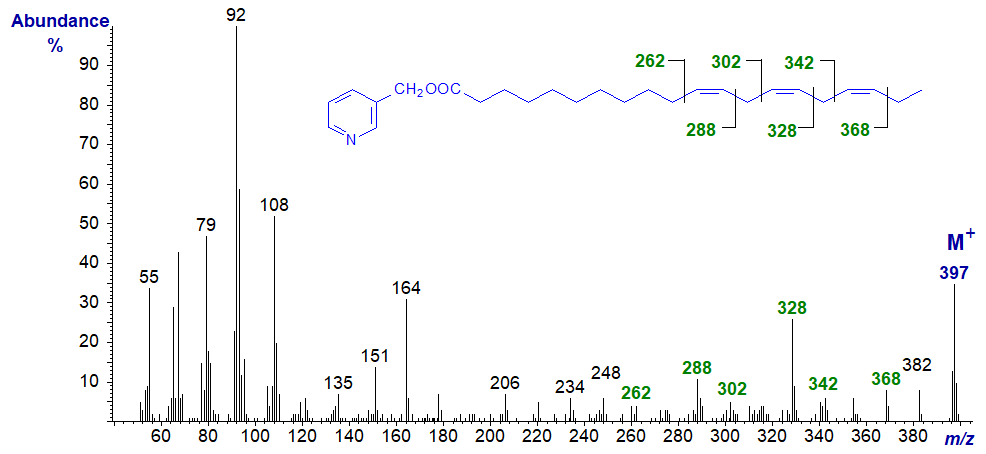
3-Pyridylcarbinyl 11,14,17-eicosatrienoate (20:3(n-3)) (from - Christie et al., 1986). With this and the previous isomer, the double bonds can be located as marked without too much difficulty. Again, note that if considered only as a 'fingerprint', each mass spectrum from the various 20:3 isomers is distinctive.
Conjugated Trienoic Fatty Acids
3-Pyridylcarbinyl 9t,11t,13t-octadecatrienoate or β-eleostearate (an occasional component of seed oils) is the only conjugated triene for which we have a spectrum, other than the alpha-isomer in which the first double bond is of the cis configuration (spectrum identical). As with conjugated dienes, the molecular ion is the base ion, but it would be difficult to locate the double bonds from first principles.
Trienoic fatty acids in which only two double bonds are in conjugation are only rarely encountered, but we have the spectrum of 3‑pyridylcarbinyl 6c,10t,12c-octadecatrienoate in the Archive section.
Bis- and Poly-Methylene-Interrupted Trienoic Fatty Acids
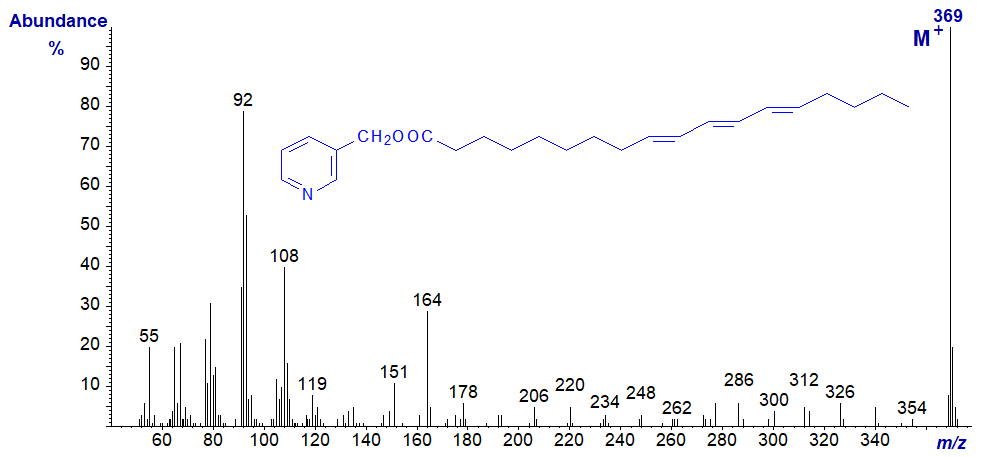
As described elsewhere for 3-pyridylcarbinol esters of comparable dienes, it has become apparent that bis- and polymethylene-interrupted trienoic fatty acids are more common in nature than may have been supposed. Fatty acids with a 5,9-double bond system or their chain elongation products are common in seed oils from Gymnosperms (conifers) or in the lipids of certain marine invertebrates such as sponges. The spectrum of 3-pyridylcarbinyl 5,9,12‑octadecatrienoate (pinolenate) from pine seeds is typical (Christie, 1989) -
As with the dienes, the abundant ion at m/z = 219 is very characteristic of a bis-methylene-interrupted double bond system in positions 5 and 9. The other ions marked provide confirmation of the double bond positions.
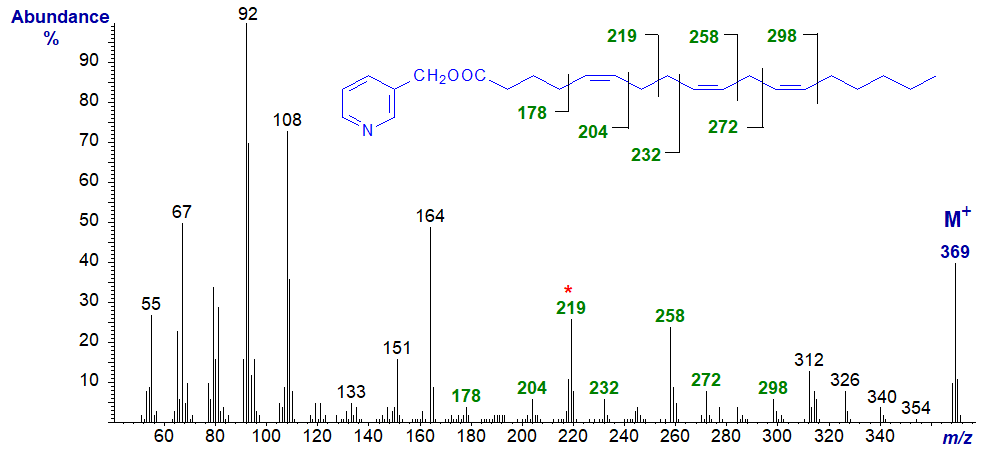
5,9,13-Eicosatrienoic acid from a sponge has two bis-methylene-interrupted double bond systems, so the mass spectrum of its 3‑pyridylcarbinol ester is especially distinctive (Joh et al., 1997)) -
In this instance, the prominent ions at m/z = 219 and 272 are diagnostic for cleavage between carbons 7 and 8 and between 11 and 12, respectively. The ions at m/z = 178, 204, 232, 258, 286 and 312 also locate the double bonds.
3-Pyridylcarbinyl 7,11,14-eicosatrienoate (from Pinaceae seed oils - Wolff et al., 1997) -
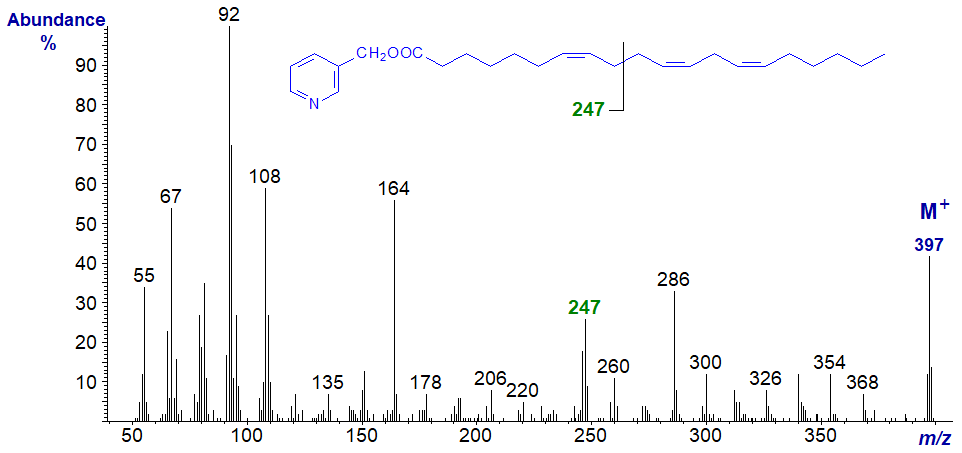
The spectrum can be considered as analogous to that of 5,9,12-18:3, with the diagnostic ions shifted 28 amu higher, i.e., the ion for the centre of the bis-methylene-interrupted double bond system is now at m/z = 247 instead of 219. The ions that locate the last two double bonds are the same as in that of pyridylcarbinyl 8,11,14-eicosatrienoate (20:3(n-6)), illustrated above.
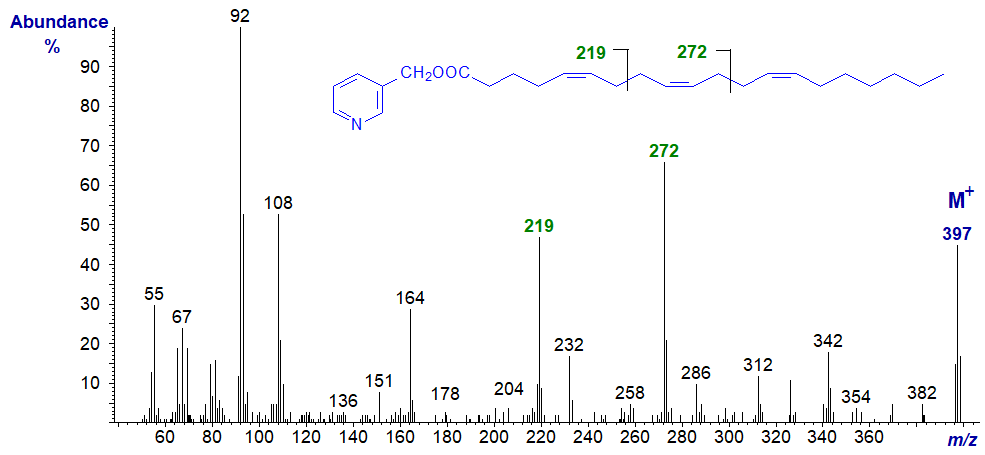
The same principles apply, even with very-long-chain fatty acids of this type, as is illustrated by the spectrum of 3-pyridylcarbinyl 5,9,25-dotriacontatrienoate (5,9,25‑32:3) -
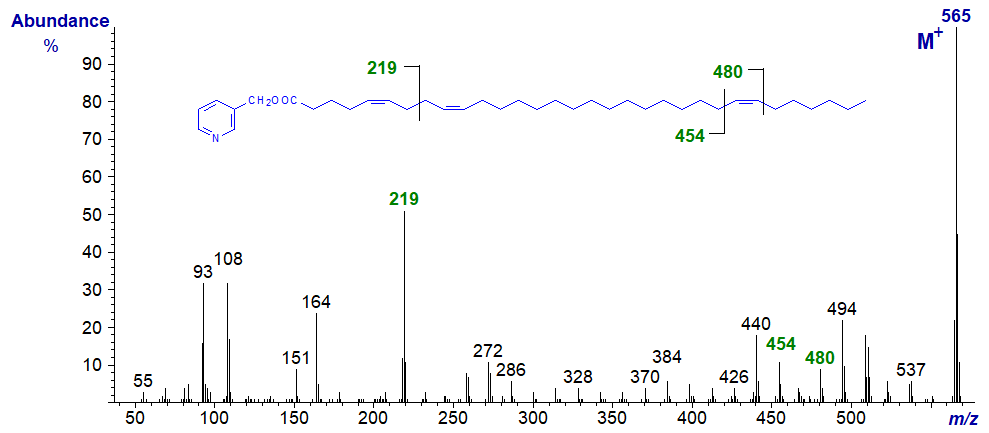
The 5,9-double bond system is indicated once more by the abundant ion at m/z = 219 with the other associated ions, while the remote double bond in position 25 is clearly indicated by the gap of 26 amu between the ions at m/z = 454 and 480.
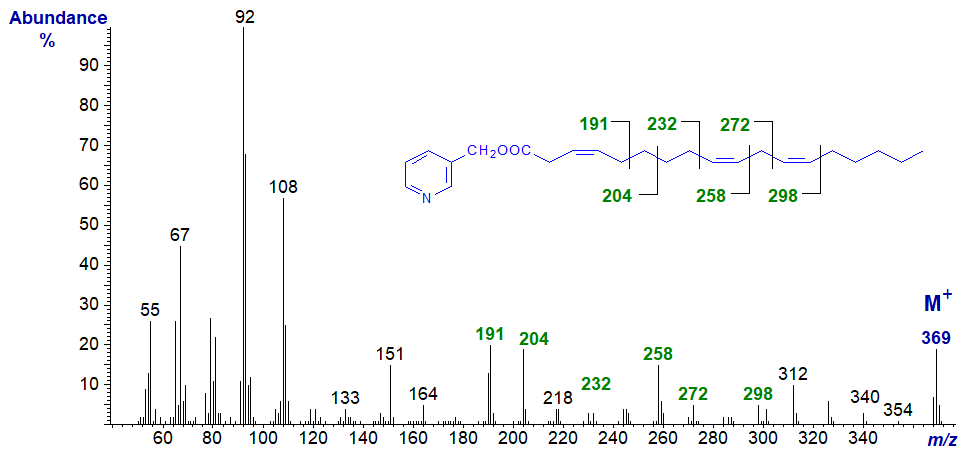
The next two spectra are of fatty acids with a double bond more than two carbon atoms from a methylene-interrupted double bond system. With 3‑pyridylcarbinyl 3,9,12-octadecatrienoate from Tanacetum zawadskii seed oil (Tsevegsuren et al., 2003), the double bonds in positions 9 and 12 are identified as for the previous spectrum, while the characteristic fingerprint ions at m/z = 190/191 and 204, formed by cleavage with allylic rearrangements, locate the remaining double bond in position 3 (see our web pages on 3‑pyridylcarbinol esters of monoenoic fatty acids).
3-Pyridylcarbinyl 5,11,14-eicosatrienoate is found in seed oils of many plants of the Gymnosperm family.
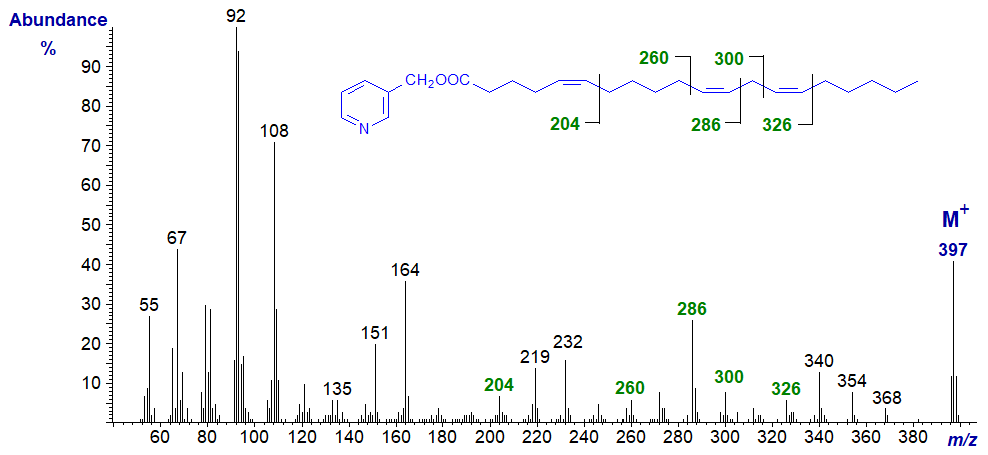
Here, interpretation is straightforward by comparison with the spectra of comparable 5-monoenes and 11,14-dienes, illustrated on the appropriate web pages, and the diagnostic ions are indicated on the spectrum.
Spectra of 3-pyridylcarbinol esters of many more trienoic fatty acids with bis-methylene-interrupted double bonds are illustrated in our Archive section, but without interpretation. Many of these have not been formally published elsewhere. Most are from sponges and range from 5,9,17-24:3 to 5,9,27‑34:3.
References
- Adkisson, H.D., Risener, F.S., Zarrinkar, P.P., Walla, M.D., Christie, W.W. and Wuthier, R.E. Unique fatty acid composition of normal cartilage: discovery of high levels of n-9 eicosatrienoic acid and low levels of n-6 polyunsaturated fatty acids. FASEB J., 5, 344-353 (1991); DOI.
- Christie, W.W. HPLC and gas chromatography-mass spectrometry in the analysis of fatty acids. In: Fats for the Future, pp. 335-344 (edited by R.C. Cambie, Ellis Horwood Ltd, Chichester) (1989).
- Christie, W.W., Brechany, E.Y., Johnson, S.B. and Holman, R.T. A comparison of pyrrolidide and picolinyl ester derivatives for the identification of fatty acids in natural samples by gas chromatography-mass spectrometry. Lipids, 21, 657-661 (1986); DOI
- Harvey, D.J. 3-Picolinyl derivatives for the structural determination of fatty acids by mass spectrometry. Applications to polyenoic acids, hydroxy acids, di-acids and related compounds. Biomed. Mass Spectrom., 11, 340-347 (1984); DOI.
- Joh, Y.G., Elenkov, I.J., Stefanov, K.L., Popov, S.S., Dobson, G. and Christie, W.W. Novel di-, tri-, and tetraenoic fatty acids with bis-methylene-interrupted double-bond systems from the sponge Haliclona cinerea. Lipids, 32, 13-17 (1997); DOI.
- Tsevegsuren, N., Fujimoto, K., Christie, W.W. and Endo, Y. Occurrence of a novel cis,cis,cis-octadeca-3,9,12-trienoic (Z,Z,Z-octadeca-3,9,12-trienoic) acid in Chrysanthemum (Tanacetum) zawadskii Herb. (Compositae) seed oil. Lipids, 38, 573-578 (2003); DOI.
- Wolff, R.L., Christie, W.W. and Coakley, D. Bishomopinolenic (7,11,14-20:3) acid in Pinaceae seed oils. J. Am. Oil Chem. Soc., 74, 1583-1586 (1997); DOI.
- Wolff, R.L., Christie, W.W., Pedrono, F. and Marpeau, A.M. Arachidonic, eicosapentaenoic, and biosynthetically related fatty acids in the seed lipids from a primitive gymnosperm, Agathis robusta. Lipids, 34, 1083-1097 (1999); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
