Mass Spectrometry of Methyl Esters
Monoenoic Fatty Acids
As cautioned in the 'Introduction' to these documents, mass spectra of methyl esters obtained with electron-impact ionization often afford limited information only concerning the structures of unsaturated fatty acids. The molecular weight is usually obtainable, and this is an important piece of information. If chromatographic retention data are added to this, it is often possible to be 90% certain of the identity of a fatty acid. Although they have a distinctive general 'fingerprint', mass spectra of methyl esters of most monoenoic fatty acids contain no information that helps to locate the position or geometry (cis v. trans) of double bonds. While there have been suggestions that such information can be obtained from close examination of certain minor ions in the spectrum, I am doubtful of the value of such techniques with real samples, as small changes in instrumental parameters, imperfect peak resolution and background noise could mask such effects. Methods of preparing methyl esters are described on another web page. There is a document on the chemistry and occurrence of monoenoic fatty acids on this site here... A few of the spectra illustrated below may have been published elsewhere, but it would be an onerous task to establish priority, and most are unique to this web site.
Straight-Chain Monoenoic Fatty Acids
The mass spectra of methyl esters of monoenoic fatty acids under electron-impact ionization tend to be rather uninformative, although they do allow the molecular weight to be determined and this together with the GC retention data are useful for identification purposes. The introduction of a double bond changes the spectrum appreciably from that of the corresponding saturated ester.
The mass spectrum of methyl oleate (cis-9-octadecenoate) is illustrated first (Hallgren et al., 1959) -
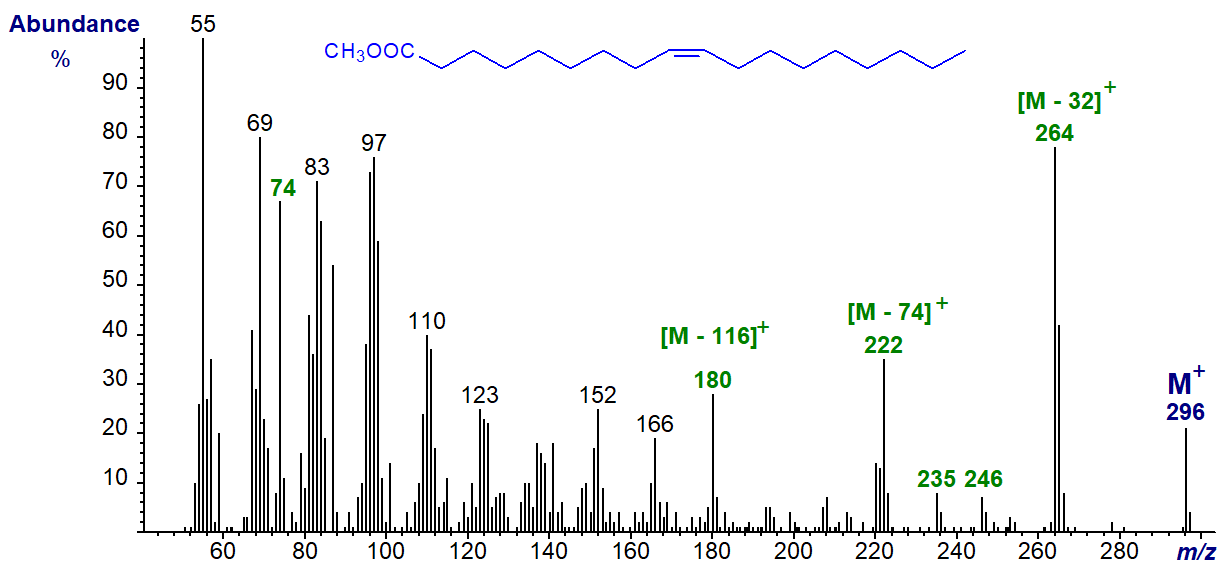
The molecular ion (m/z = 296) is clearly seen, and ions representing loss of the elements of methanol (m/z = 264 or [M-32]+), i.e., a methoxyl group plus a hydrogen atom, and the loss of the McLafferty ion (m/z = 222) are abundant, as is the McLafferty ion per se (m/z = 74) (see the web page on mass spectra of methyl esters of saturated acids). Smaller ions equivalent to [M‑60/61]+ and [M‑49/50]+ at m/z = 235 and 246, respectively in this instance, are present here and in the spectra of most other isomers. A characteristic ion at [M-116]+ (m/z = 180 in this instance), together with homologous ions at 166, 152, etc., are also diagnostic. That at [M‑116]+ is formed by loss of a fragment containing the carboxyl group by cleavage between carbons 5 and 6 with addition of a rearranged hydrogen atom. Thus, in contrast to the spectra of methyl esters of saturated fatty acids, hydrocarbon ions (general formula [CnH2n‑1]+) dominate the spectrum, with m/z = 55 as the base ion usually. The relative abundances of many of these ions tend to be appreciably greater than in the mass spectra of dienes and polyenes.
There is no feature that permits location of the double bond because this can migrate to any position when the alkyl chain is ionized in the mass spectrometer. Thus, other than when the double bond is adjacent to the carboxyl group, all the cis- and trans-18:1 isomers have very similar spectra. The same ions are present, and while the relative intensities can be variable, especially in the high mass region, the fingerprints are not sufficiently distinctive to be of value diagnostically, other than with the 2- and possibly 3-isomers. Note that identifications of such monoenes by using computerized data bases are highly unreliable.
The main exception is the spectrum of methyl 2-octadecenoate (below) in which the double bond and carboxyl group presumably form a relatively stable resonance structure. For example, there is a distinctive ion at m/z = 113, which is believed to occur via a cleavage between carbons 5 and 6 followed by formation of a stable 6‑membered ring with the carboxyl group (Ryhage et al., 1961). The base ion is at m/z = 87 in this instance.
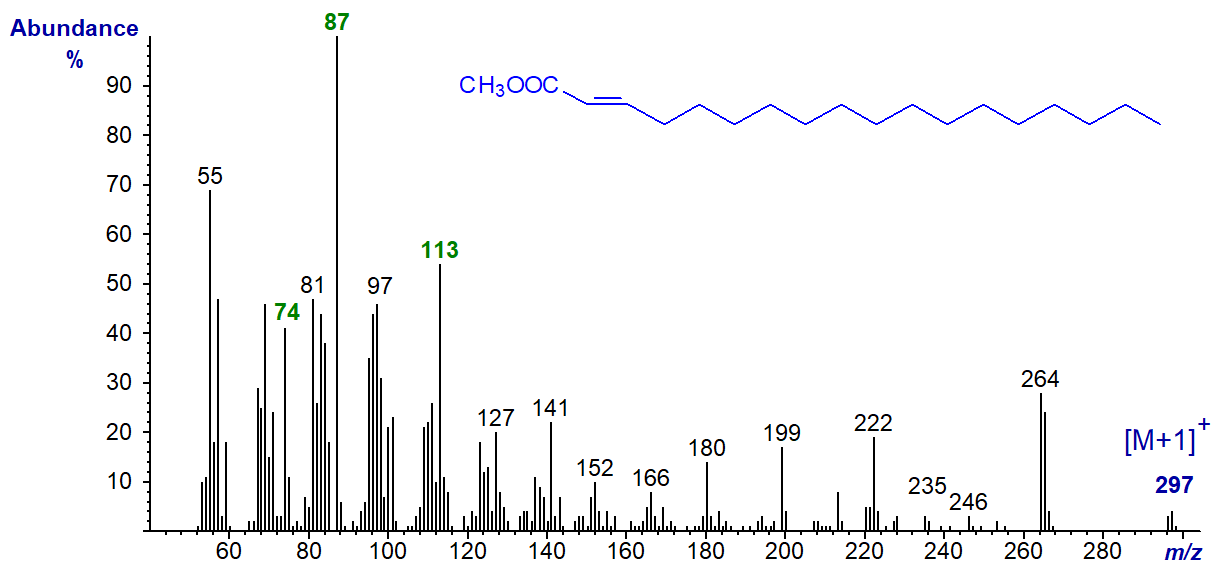
This fatty acid does not occur naturally to my knowledge, but it can be formed artefactually by over vigorous trans-esterification of the 3‑isomer. However, some natural fatty acids with α,β-unsaturation in addition to other functional groups are known.
Trans-3-octadecenoic acid (trans-3-18:1) and trans-3-16:1 occur naturally in photosynthetic tissues of plants and some seed oils, and the spectrum of methyl ester is of the former is -
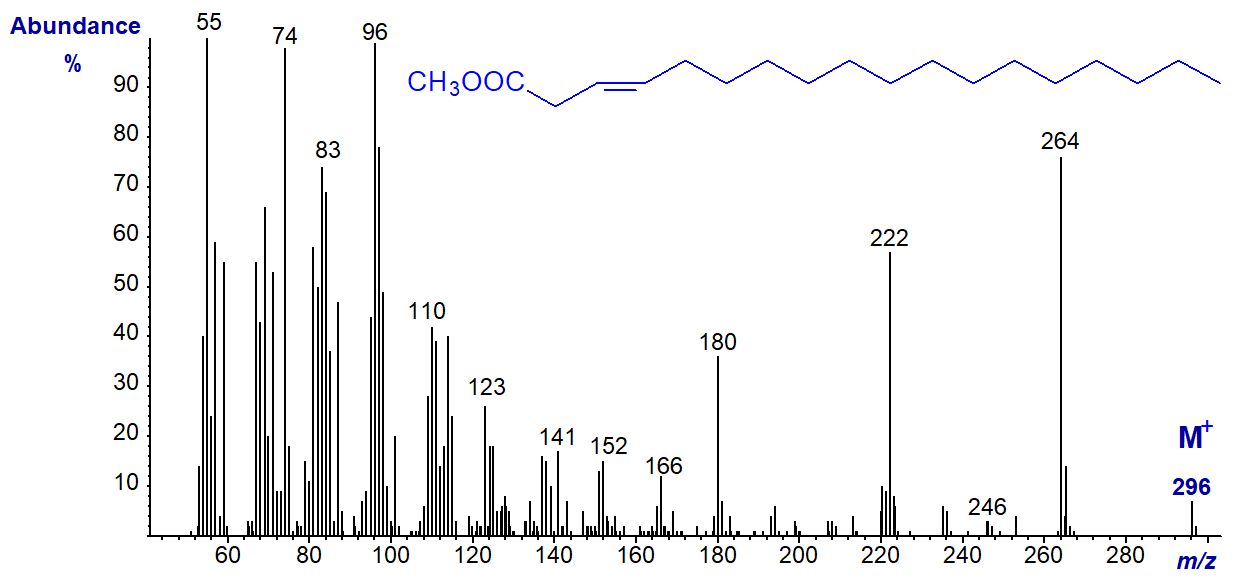
There are minor differences in the relative abundances of some ions relative to the spectrum of methyl oleate, and the author has found the fingerprint useful for confirmatory purposes in analyses of certain seed oils. In addition, the GC retention time relative to those of other 18:1 isomers is a further aid to identification.
As representatives of monoenes of other chain-lengths, first the mass spectrum of methyl 5-eicosenoate (from meadowfoam oil) is -
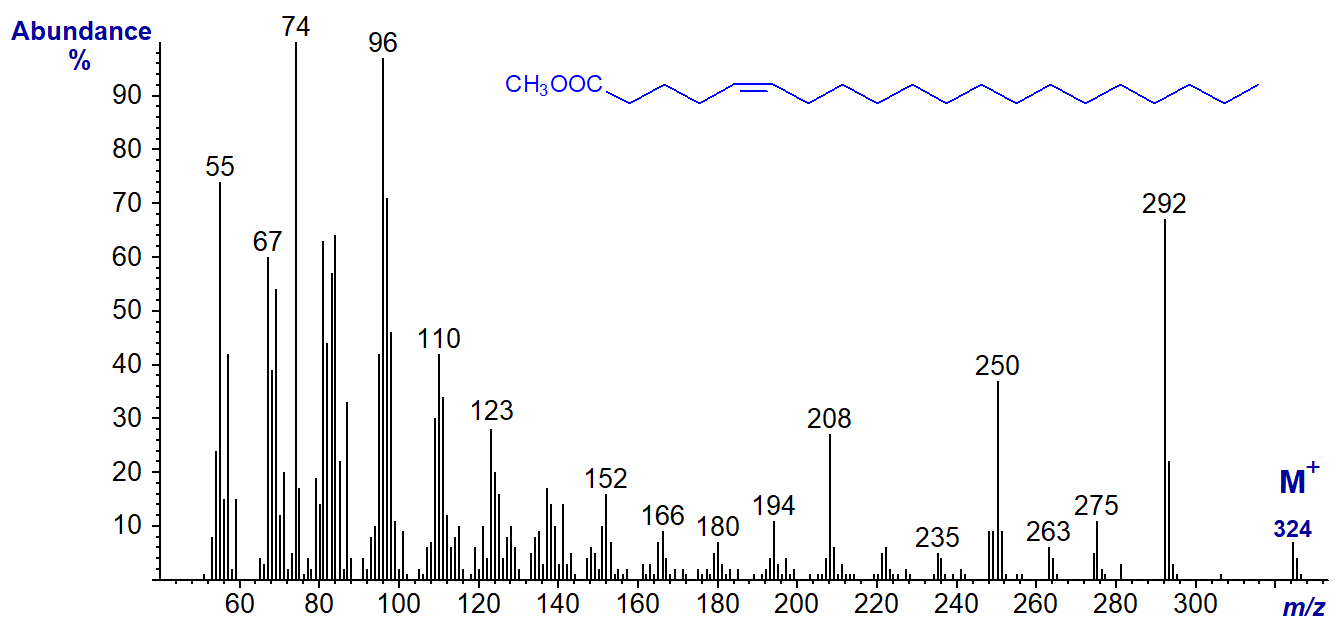
This and the next spectrum have comparable ions to those for methyl oleate, especially the distinctive ions for [M-32]+, [M-74]+ and [M‑116]+, which are now at m/z = 292, 250 and 208, respectively.
The spectrum of methyl 13-docosenoate (also from meadowfoam oil) is -
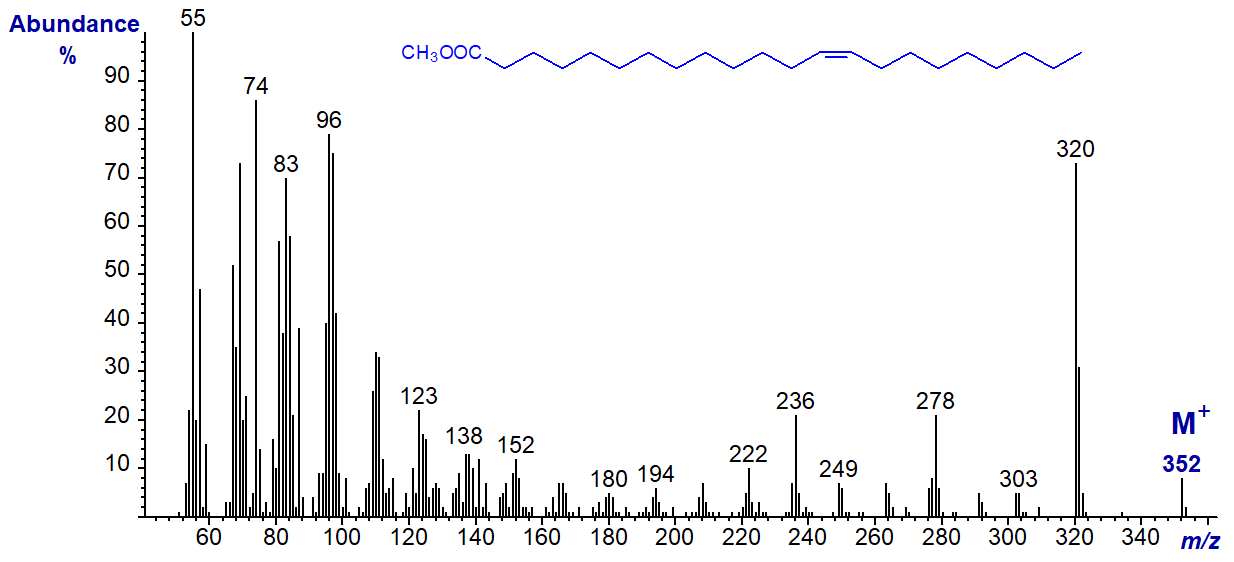
Again, the ions representing [M-32]+, [M-74]+ and [M-116]+ at m/z = 320, 278 and 236, respectively, stand out.
The mass spectrum of the shorter-chain methyl 9-dodecenoate (from milk fat) is –
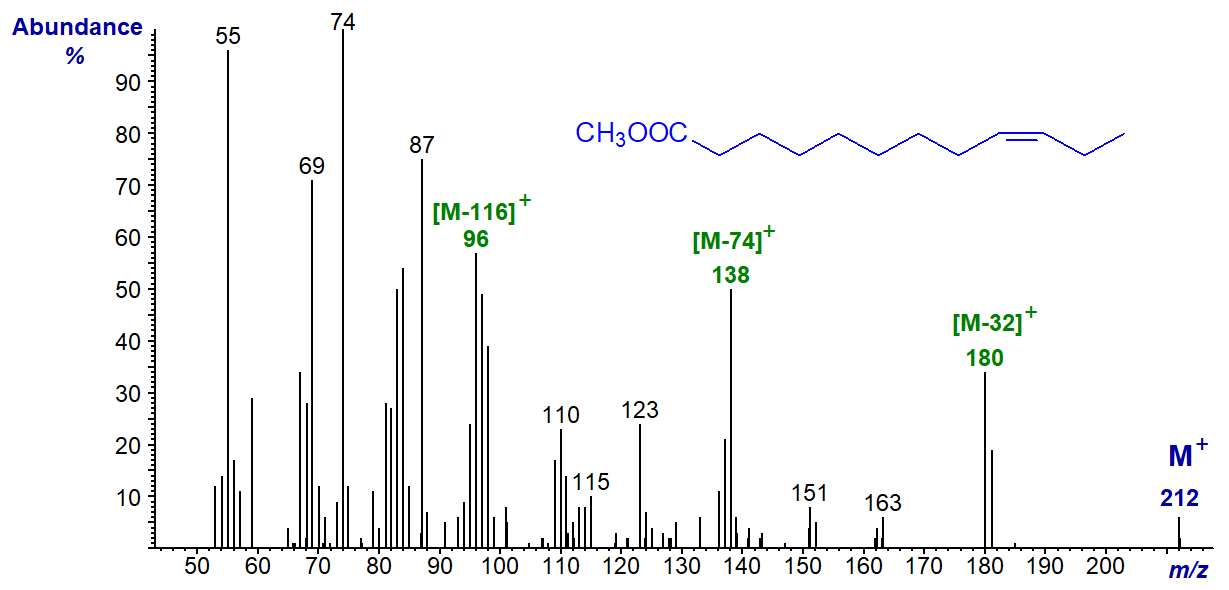
Analogous ions to those in the previous spectra are present, although the relative intensities are somewhat different as might be expected in view of the very different chain length.
Note that the presence of an alicyclic ring reduces the molecular weight of a fatty acid by 2 amu in comparison to the corresponding straight-chain saturated fatty acid, i.e., the same amount as one double bond. While this is not a problem with larger ring structures (5 to 6), especially when at the end of the chain, methyl esters of cyclopropyl fatty acids have mass spectra that are virtually identical to those of monoenes with the same total number of carbon atoms (see the web page on Mass spectra of methyl esters of cyclic fatty acids). Where there is doubt, GC retention data are an important aid to identification.
Branched-Chain Monoenoic Fatty Acids
Methyl branches in saturated fatty acids produce distinctive cleavages in the mass spectra that usually permit identification (see the relevant web page), but few authentic spectra are available from branched-chain monoenes to allow anything other than speculation in interpretation. Unsaturated branched-chain fatty acids are occasionally encountered as minor components of marine lipid samples, and the mass spectrum of methyl 15-methyl-hexadec-9-enoate (from a sponge) is -
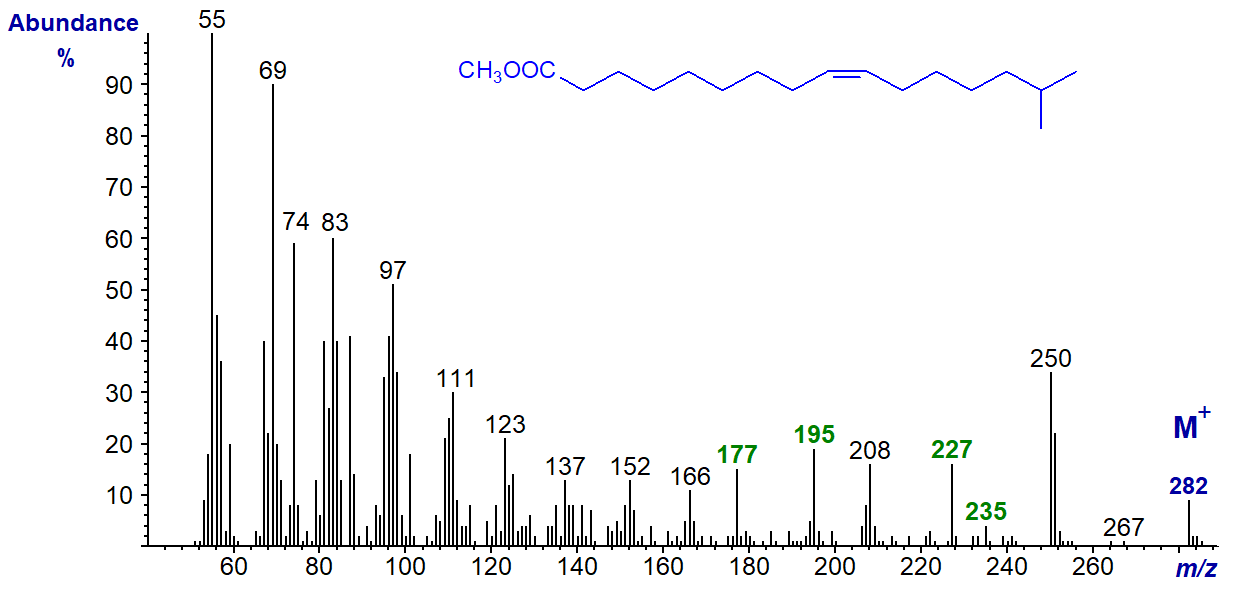
The ions at m/z = 235 ([M-47]+) and 227 ([M-55]+) and presumably those at m/z = 177 and 195 may be related in an as yet undefined way to the presence of the iso‑methyl group, perhaps influenced by the presence of the double bond. Until this is clarified, the spectrum should be regarded as simply as a fingerprint.
The mass spectrum of methyl 7-methyl-hexadec-6-enoate (from a jellyfish in this instance, but often present as a minor component in fish from warmer seas) is -
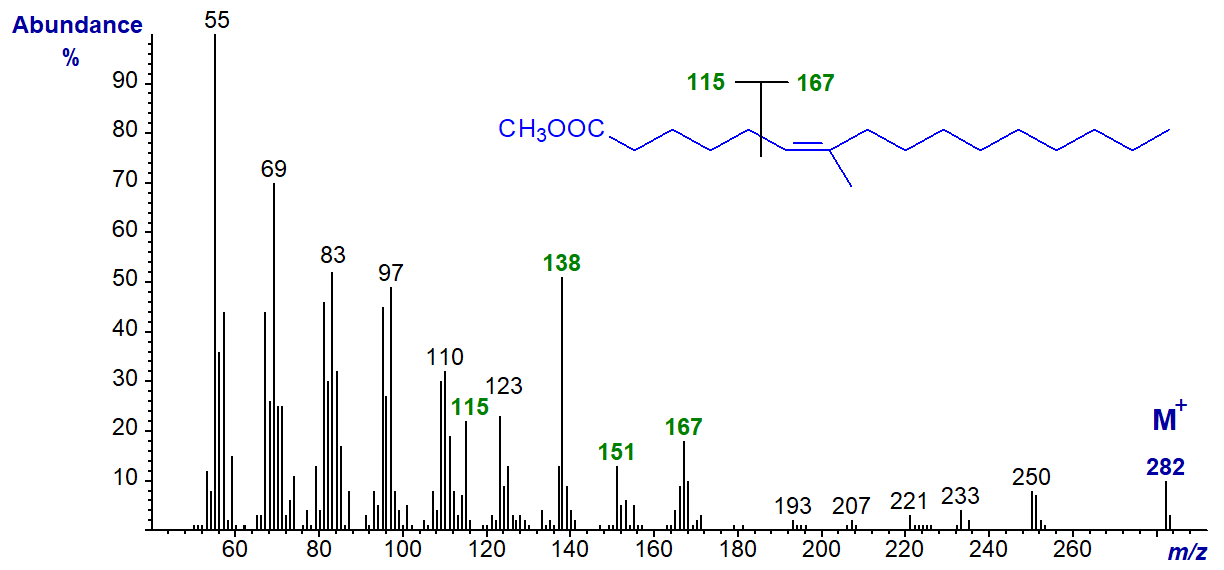
In contrast to the spectra of other monoenes, ions representing [M-32]+, [M-74]+ and [M-116]+ are small. It is tempting to suggest that the ions at m/z = 115 and 167 result simply from fragmentations between carbons 5 and 6 as illustrated. Evidence supporting this interpretation comes from the spectrum of the ethyl ester, where the ion at m/z = 167 remains, but that at m/z = 115 is replaced by one at m/z = 129. After publication here, this spectrum was illustrated in a paper by Fardin-Kia, A.R. et al. (2013), together with that of methyl 7-methyl-octadec-6-enoate in which the ion at m/z = 167 is replaced by one at m/z = 195, as might be expected if the above interpretation is correct. The distinctive ions at m/z = 138 and 151 are present in the spectra both of the ethyl ester and of the 7-methyl-18:1 analogue, so they are presumed to result from a rearrangement of the carboxyl end of the molecule (with loss of at least a methoxyl/ethoxyl moiety).
We have the mass spectrum of the monoenoic isoprenoid methyl 3,7,11,15-tetramethylhexadec-trans-2-enoate (phytenate) on file in the Archive Section, but any interpretation of the fragmentation pattern would be speculative.
Alternative methodology: To get round the problem of location of double bonds in monoenes at least, it is possible to prepare specific derivatives of unsaturated fatty acids that 'fix' the double bond. Many have been described, but the only one to have stood the test of time is the dimethyl disulfide adduct, as this has excellent mass spectrometric properties and is prepared in a simple one-pot reaction (see the section on 'Mass spectra of methyl esters of fatty acids - further derivatization'). A useful feature is that cis- and trans-isomers are separable. Alternatively, 3‑pyridylcarbinol (‘picolinyl’) esters or DMOX or pyrrolidine derivatives can be utilized to locate double bonds, as described in some detail elsewhere in these web pages. Mass spectrometry with chemical ionization and acetonitrile as the reagent gas can enable both the position and geometry of a double bond to be determined, as reviewed by Brenna (2006), but as I have no personal experience of this methodology I cannot comment further.
Spectra of many more methyl esters of monoenoic fatty acids can be accessed from our Archive pages (without interpretation).
References
- Brenna, J.T. Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent adduct chemical ionization. In: Lipid Analysis and Lipidomics: New Techniques and Applications. pp. 157-172 (edited by: M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, USA) (2006).
- Fardin-Kia, A.R., Delmonte, P., Kramer, J.K.G., Jahreis, G., Kuhnt, K., Santercole, V. and Rader, J.I. Separation of the fatty acids in menhaden oil as methyl esters with a highly polar ionic liquid gas chromatographic column and identification by time of flight mass spectrometry. Lipids, 48, 1279-1295 (2013); DOI.
- Hallgren, B., Ryhage, R. and Stenhagen, E. The mass spectra of methyl oleate, methyl linoleate and methyl linolenate. Acta Chem. Scand., 13, 845-847 (1959); DOI.
- Ryhage, R., Ställberg-Stenhagen, S. and Stenhagen, E. Methyl esters of α,β-unsaturated long-chain acids. On the structure of C27-phthienoic acid. Arkiv. Kemi, 18, 179-186 (1961).
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
