Introduction to Mass Spectrometry of Fatty Acids
and These Web Pages
What You Will Find in These Web Pages
This series of online tutorials is intended to be a practical guide to determining the structures of natural fatty acids by gas chromatography linked to mass spectrometry (GC-MS) with electron-impact ionization and is a personal account based on my own practical experience. Far more mass spectra are illustrated than would be possible in a conventional review - over 700 at the last count - and this does not include our Archive pages where more than 2,200 mass spectra are displayed (without interpretation). To see the latter, visit our Archive of methyl esters --- 3‑pyridylcarbinol ('picolinyl') esters --- DMOX derivatives --- pyrrolidides --- other esters and miscellaneous lipids. I hope the adage that "a picture is worth a thousand words" applies here, because it is not possible to rely on computerized identifications of mass spectra unless this is backed by a sufficient understanding of the principles of mass spectrometry.
 I have tried to illustrate spectra of C18 fatty acids
in these tutorials wherever possible to make comparisons easier, but fragmentation patterns can usually be extrapolated to other chain-lengths.
These web pages have been divided into six main sections, starting with basic information on selection and preparation
of derivatives both at carboxyl groups and double bonds, together with some useful ancillary techniques.
Then, there are sections dealing with mass spectra of the main derivative types, methyl esters,
3-pyridylcarbinol ('picolinyl') esters, pyrrolidides and 4,4-dimethyloxazolines (DMOX).
In the final section, spectra of fatty acids in the form of esters other than methyl are described
together with those of some miscellaneous lipids encountered in our research, e.g., aliphatic acetals, alcohols and sterols.
Some contaminants that may be encountered in fatty acid preparations are also discussed.
I have tried to illustrate spectra of C18 fatty acids
in these tutorials wherever possible to make comparisons easier, but fragmentation patterns can usually be extrapolated to other chain-lengths.
These web pages have been divided into six main sections, starting with basic information on selection and preparation
of derivatives both at carboxyl groups and double bonds, together with some useful ancillary techniques.
Then, there are sections dealing with mass spectra of the main derivative types, methyl esters,
3-pyridylcarbinol ('picolinyl') esters, pyrrolidides and 4,4-dimethyloxazolines (DMOX).
In the final section, spectra of fatty acids in the form of esters other than methyl are described
together with those of some miscellaneous lipids encountered in our research, e.g., aliphatic acetals, alcohols and sterols.
Some contaminants that may be encountered in fatty acid preparations are also discussed.
My approach to these web pages was made possible by the wide range of mass spectra available to me and gathered over 40 years of research in lipid chemistry. We have spectra of methyl esters of more than 500 different fatty acids on file, as well as those for more than 500 3‑pyridylcarbinol esters, 320 DMOX derivatives, 250 pyrrolidides and many other fatty acid derivatives (and related lipids), again more than 500 spectra, some of which have been supplied by friends and collaborators. Uniquely, we have spectra of all the possible cis-18:1 isomers, from 2‑18:1 to 17‑18:1, as 3‑pyridylcarbinol esters, DMOX derivatives and pyrrolidides, and for all the methylene-interrupted 18:2 isomers (2,5‑18:2 to 14,17‑18:2), and these are illustrated here. To this can be added, branched-chain, cyclic, oxygenated, sulfur-containing, halogenated, allenic, non-methylene-interrupted dienes and polyenes, and many others.
Mechanistic aspects are covered only briefly in these documents, and fragmentations are usually illustrated simplistically although some basic knowledge of this topic can be invaluable. As I have cautioned elsewhere, it is unwise to rely too much on computerized identifications of fatty acid spectra using any of the commercial data bases.
Why Gas Chromatography-Mass Spectrometry is Invaluable
The common fatty acids of animal and plant origin have even-numbered chains of 16 to 22 carbon atoms with zero to six double bonds of the cis configuration with methylene-interrupted double bond systems predominating, but Nature provides countless exceptions, and odd- and even-numbered fatty acids with up to nearly a hundred carbon atoms exist. In addition, double bonds can be of the trans configuration, acetylenic and allenic bonds occur, and there can be innumerable other structural features, including branch points, rings, oxygenated functions and many more. My best estimate is that 2000 different fatty acids of natural origin must exist, as well as others produced as artefacts, for example by autoxidation.
 In
many research fields, it is essential to have simple rapid methods for determination of fatty acid structures
and for isolation of pure components of mixtures for further analysis.
GC-MS with electron-impact ionization is especially useful for characterization purposes, and the simplest commercial bench-top instruments
are well suited to the purpose.
A feature of special importance with GC-MS is that it is rarely necessary to isolate components in a pure form,
as may be required for other spectroscopic methods (e.g., NMR spectroscopy) or for chemical degradative procedures.
The instrumentation is much less costly and simpler to use and maintain than modern LC-MS equipment, such as
that with electrospray ionization, which is making such an impact on so many other fields of lipid research.
In
many research fields, it is essential to have simple rapid methods for determination of fatty acid structures
and for isolation of pure components of mixtures for further analysis.
GC-MS with electron-impact ionization is especially useful for characterization purposes, and the simplest commercial bench-top instruments
are well suited to the purpose.
A feature of special importance with GC-MS is that it is rarely necessary to isolate components in a pure form,
as may be required for other spectroscopic methods (e.g., NMR spectroscopy) or for chemical degradative procedures.
The instrumentation is much less costly and simpler to use and maintain than modern LC-MS equipment, such as
that with electrospray ionization, which is making such an impact on so many other fields of lipid research.
Fatty acids are most often analysed routinely by GC as methyl ester derivatives, but mass spectra of these may not always contain ions indicative of key structural features; the positions of double bonds in the aliphatic chain can only rarely be determined unequivocally. That said, useful 'fingerprint' spectra are often obtainable with polyunsaturated derivatives to enable identification by comparison with standard spectra. Indeed, there are many occasions when it is convenient to analyse fatty acid methyl esters by mass spectrometry, such as for confirmatory purposes or as a guide to what further work may be required. Molecular weight and GC retention times are useful analytical data from such analyses, some limited structural information may be available, and indeed definitive spectra can be obtained often from fatty acids with branched-chains, cyclic structures or additional oxygenated functional groups. Methyl esters usually give better resolution on most GC columns than other derivatives of components that otherwise might overlap.
In a more useful approach to structure determination, the carboxyl group is derivatized with a reagent containing a nitrogen atom. When the molecule is ionized in the mass spectrometer, the nitrogen atom not the alkyl chain carries the charge, and double bond ionization and migration is minimized. Radical-induced cleavage occurs evenly along the chain and gives a series of relatively abundant ions of high mass from the cleavage of each C-C bond. When a double bond or other functional group is reached, diagnostic ions are usually produced. The first useful nitrogen-containing derivatives, i.e., N-acyl pyrrolidides, were described fifty years ago; they give useful spectra and should not be discounted (indeed I believe that they have been greatly under-valued for labile fatty acids, such as those with epoxide rings, or with terminal functional moieties). However, most analysts now prefer either 3‑pyridylcarbinol ester or 4,4‑dimethyloxazoline (DMOX) derivatives.
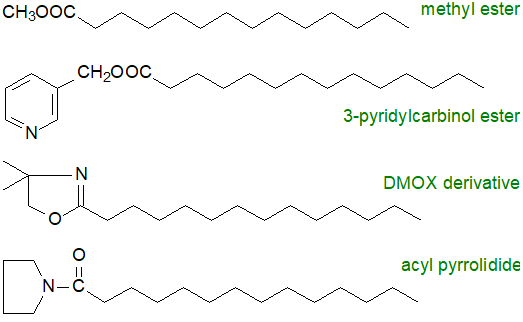 |
| Figure 1. Derivatives for mass spectrometry of fatty acids. |
Both 3‑pyridylcarbinol esters and DMOX derivatives have their merits in mass spectrometry terms, and neither should be neglected. Each has advantages for certain types of fatty acid, and they are best considered as providing complementary information rather than simply as alternatives. With difficult samples in my own research, I have prepared both types of derivative together often with pyrrolidides. As methyl esters are usually available for other purposes, such as for more accurate quantification, it is often convenient to obtain their spectra if only for confirmation or as a record. 3‑Pyridylcarbinol esters and pyrrolidides tend to give spectra that are easier to interpret than those of DMOX derivatives when functional groups are near the terminal end of the fatty acyl chain (Hamilton, J.T.G. and Christie, W.W., 2000). On the other hand, DMOX derivatives may have advantages for functional groups in positions 4 to 6.
 In choosing a derivative for mass spectrometry, good chromatographic properties
are important, and it is always helpful when straightforward derivatization procedures can be recommended that use readily available reagents
and have simple glassware requirements.
One advantage of DMOX derivatives is that they are only slightly less volatile than methyl esters; they can be subjected to GC analysis on polar
stationary phases under similar conditions and can often give comparable resolution.
Pyrrolidides may not have as good chromatographic properties, but they can give some unexpected separations.
3‑Pyridylcarbinol esters, on the other hand, require column temperatures about 50°C higher than for methyl esters
and that meant initially that they had to be separated on non-polar phases, such as DB‑5TM, which gave relatively poor resolution.
With the introduction of new polar phases, which are stable to high column temperatures and have low-bleed characteristics for MS analysis,
such as BPX-70TM or even some of those of the Carbowax type, such as Supelcowax 10TM, the problem of GC resolution of
3‑pyridylcarbinol esters is greatly lessened and only very-long-chain fatty acids (>C24) tend to cause problems.
From time to time, we still find a DB-5TM column to be of value for fatty acids of high molecular weight (and for sterols, etc.), and
no doubt other GC phases are currently available of which we have no practical experience.
We have a separate web page that discusses the merits of different types of GC columns in more detail.
In choosing a derivative for mass spectrometry, good chromatographic properties
are important, and it is always helpful when straightforward derivatization procedures can be recommended that use readily available reagents
and have simple glassware requirements.
One advantage of DMOX derivatives is that they are only slightly less volatile than methyl esters; they can be subjected to GC analysis on polar
stationary phases under similar conditions and can often give comparable resolution.
Pyrrolidides may not have as good chromatographic properties, but they can give some unexpected separations.
3‑Pyridylcarbinol esters, on the other hand, require column temperatures about 50°C higher than for methyl esters
and that meant initially that they had to be separated on non-polar phases, such as DB‑5TM, which gave relatively poor resolution.
With the introduction of new polar phases, which are stable to high column temperatures and have low-bleed characteristics for MS analysis,
such as BPX-70TM or even some of those of the Carbowax type, such as Supelcowax 10TM, the problem of GC resolution of
3‑pyridylcarbinol esters is greatly lessened and only very-long-chain fatty acids (>C24) tend to cause problems.
From time to time, we still find a DB-5TM column to be of value for fatty acids of high molecular weight (and for sterols, etc.), and
no doubt other GC phases are currently available of which we have no practical experience.
We have a separate web page that discusses the merits of different types of GC columns in more detail.
It is worth noting that methyl esters, 3‑pyridylcarbinol esters and pyrrolidide derivatives are prepared under relatively mild conditions, and they are stable chemically so can be stored for long periods at –20°C. The most widely used method for DMOX derivatives may require harsher conditions, and precautions are necessary to prevent hydrolysis on storage, but at least it is a simple one-pot method that can be applied to most lipid types; milder methods are now available when required. Preparation of the nitrogen-containing derivatives and of methyl esters are described in detail on separate web pages. Of course, there are times when it is necessary to prepare additional derivatives of functional groups other than the carboxyl, and for example, trimethylsilyl ethers can aid the analysis of hydroxy fatty acids.
Other chromatographic and spectroscopic methods are available as an aid to characterization. In particular, high-performance liquid chromatography in the reversed-phase and silver ion modes are our favourite procedures for simplifying mixtures prior to GC-MS (see our web page on this aspect of the topic).
Alternative Mass Spectrometric Techniques
Many alternative types of fatty acid derivative to those discussed in these pages have been described in the scientific literature, but as we have no experience of them, we cannot discuss them here. Any new derivatives described should have a better combination of chromatographic and mass spectrometric fragmentation properties than the existing ones if they are to be taken seriously. Thus, while simple oxazoline derivatives, as opposed to the dimethyloxazolines, look excellent on paper, there is no body of published spectra for comparison purposes (Kuklev, D.V. and Smith, W.L., 2003).
Mild ionization procedures have their place, but I have little practical experience and no spectra available for illustrative purposes. To compensate in part, I have listed some publications below that exemplify what is possible. Some very interesting papers have appeared dealing with acetonitrile-chemical-ionization tandem mass spectrometry in the gas phase for locating double bonds in fatty acid methyl esters mainly from the laboratory of Professor J. Thomas Brenna and colleagues, and this technique has the virtue of being able to distinguish cis/trans isomers. A bibliography of relevant published papers is available on this website. Some interesting results have been obtained by direct infusion or ‘shotgun’ methods, but they seem only suitable for rapid screening in my estimation at least. That said, derivatization of fatty acids with N-(4-aminomethylphenyl) pyridinium (AMPP) in conjunction with these techniques looks promising. Similarly, I have no experience of remote-site fragmentation methods involving tandem mass spectrometry or collisional activation of carboxylate anions or alkali metal-cationized fatty acids, although they may be be useful for locating double bonds.
These methods and the other mild ionization techniques often require more sophisticated and expensive instrumentation than the electron-impact ionization methods described here, and they rely heavily on computerized analysis of the data. While liquid chromatography-MS with electrospray ionization is being used increasingly for identification of fatty acid derivatives of various kinds, it may offer no technical advantages over GC-MS for analysis of the common range of fatty acids that are likely to be encountered in animal and plant tissues. On the other hand, it may undoubtedly be the optimum approach for many oxylipins, which are relatively unstable chemically, or for fatty acid derivatives of higher molecular weight. Again, I have no mass spectra obtained by such techniques to reproduced here, and I cannot comment further. Mass spectrometry is now being used determine double bond positions in fatty acids while they are esterified in intact lipids, but my impression is that this is only suitable for a limited range of fatty acids in mammalian samples.
Quantitative Analysis
I have NOT described the use of mass spectrometry for quantitative analysis of fatty acids in these web pages. Then, quite different problems arise, especially a need for careful calibration with appropriate standards, which may not be available; methyl ester derivatives may be as good as any other for this purpose. In my opinion, gas chromatography (GC) with flame-ionization detection is by far the simplest and arguably the most accurate approach to quantitative analysis when a sample contains the mainstream range of fatty acids. Oxylipins require very different treatment, for which I have no experience or relevant spectra.
References and Recommended Reading
For the references cited above and for general information on fatty acid analysis or lipid analysis in general, I recommend that readers consult my book - Lipid Analysis (4th Edition) - and the review articles listed below, some of which discuss mechanistic aspects of mass spectrometric fragmentations in greater detail than is appropriate here.
- Brenna, J.T. Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent adduct chemical ionization. In: Lipid Analysis and Lipidomics: New Techniques and Applications. pp. 157-172 (ed: M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, USA) (2006).
- Chen, C., Li, R.J. and Wu, H. Recent progress in the analysis of unsaturated fatty acids in biological samples by chemical derivatization-based chromatography-mass spectrometry methods. J. Chromatogr. B, 1215, 123572 (2023); DOI.
- Christie, W.W. Structural analysis of fatty acids. In Advances in Lipid Methodology - Four, pp. 119-169 (1997) (edited by W.W. Christie, Oily Press, Dundee).
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Kuklev, D.V. and Smith, W.L. A procedure for preparing oxazolines of highly unsaturated fatty acids to determine double bond positions by mass spectrometry. J. Lipid Res., 44, 1060-1066 (2003); DOI.
- Murphy, R.C. Mass Spectrometry of Lipids (Handbook of Lipid Research, Vol. 7) (Plenum Press, N.Y.) (1993).
- Quehenberger, O., Armando, A.M. and Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta, Lipids, 1811, 648-656 (2011); DOI.
- Serrano, R., Navarro, J.C., Portoles, T., Sales, C., Beltran, J., Monroig, O. and Hernandez, F. Identification of new, very long-chain polyunsaturated fatty acids in fish by gas chromatography coupled to quadrupole/time-of-flight mass spectrometry with atmospheric pressure chemical ionization. Anal. Bioanal. Chem., 413, 1039–1046 (2021); DOI.
- Watrous, J.D. and 14 others. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem. Biol., 26, 433-442 (2019); DOI.
Murphy's book is most useful for those interested in mechanistic aspects of mass spectrometry of lipids with electron-impact ionization. Of course, there are many specialist books available that deal with the principles of mass spectrometry in general, but I cannot offer specific guidance as my library access is now limited. Many more review articles are listed in our Bibliography - review articles document, but readers will find that these web pages contain much more practical information and vastly more illustrations of mass spectra than are available in any more formal publication.
Sources
I must gratefully acknowledge that most of the spectra on this web site have been obtained as part of the lipid chemistry research effort at the James Hutton Institute (Dundee, Scotland), and few of these have been illustrated elsewhere. Only a small number of these are available in the commercial mass spectrometry libraries (and then I have often been the primary source). I am grateful to a number of scientists who have provided samples for analysis or mass spectrometry files obtained on Agilent instruments (for which I have appropriate software), and they are acknowledged in the relevant web pages as well as in our Credits page. An extensive bibliography is provided in separate documents in the various sections of this website, listing more than a thousand relevant references, but I have kept citations in the text to an essential minimum, as it is not always possible to establish priority of publication for individual mass spectra. These pages are revised and improved whenever new information and spectra become available.
| © Author: William W. Christie |  |
|
| Updated: February 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
