Preparation of Methyl Esters for Mass Spectrometry
1. Introduction
Although methyl esters are not the best derivatives for the mass spectrometric identification of fatty acids, they are so simple in structure and in such wide-spread use for fatty acid analysis in general that they are inevitably much used for mass spectrometry. Without further derivatization, they are of limited value for structural analysis of mono- or dienoic fatty acids, but they can often be useful for polyunsaturated fatty acids and for those with functional groups other than double bonds, especially when spectra of authentic compounds are available for comparison purposes. The molecular weight is usually obtainable, and this is an important piece of information. If gas chromatographic retention data are added to this, it is often possible to be 90% certain of the identity of a fatty acid. In addition, the positions of structural features such as branch points and oxygenated groups can usually be deduced from mass spectra. Following a brief description of the methodology, experimental protocols for the two methods used most often for preparing methyl esters from lipid samples are given here.
 More detailed
discussion of the mechanisms of esterification and alternative methods are given in a substantial review article [1 - with link to pdf file]
or in my book [2].
Other useful derivatization techniques for use with methyl esters, e.g., hydrogenation, deuteration, etc., are described in the section of
these pages dealing with
'Mass spectrometry of methyl esters - derivatization of double bonds'.
Similarly, full practical details are given elsewhere on this site for preparation of the
nitrogen-containing derivatives, i.e., 3-pyridylcarbinol esters, 4,4-dimethyloxazoline (DMOX) derivatives and pyrrolidides,
which are most useful for mass spectrometric analysis of fatty acids here...
More detailed
discussion of the mechanisms of esterification and alternative methods are given in a substantial review article [1 - with link to pdf file]
or in my book [2].
Other useful derivatization techniques for use with methyl esters, e.g., hydrogenation, deuteration, etc., are described in the section of
these pages dealing with
'Mass spectrometry of methyl esters - derivatization of double bonds'.
Similarly, full practical details are given elsewhere on this site for preparation of the
nitrogen-containing derivatives, i.e., 3-pyridylcarbinol esters, 4,4-dimethyloxazoline (DMOX) derivatives and pyrrolidides,
which are most useful for mass spectrometric analysis of fatty acids here...
There is no need to hydrolyse lipids to obtain the free fatty acids before preparing esters, although this is occasionally recommended by organizations that set standards, as most lipids can be transesterified directly. As there is no single reagent that is suitable for every purpose, one must be chosen that best fits the circumstances. The two main procedures are acid- or base-catalysed esterification/transesterification. Diazomethane was once used frequently as a mild method for esterification of free acids, but concerns over the toxicity of reagents now limit its use. While the methods described below are suitable for the common esterified lipids and their fatty acid constituents, special methods are required for amide-bound fatty acids (sphingolipids) and for those of short chain-length or with sensitive functional groups for which the review and book cited below should be consulted. Esters of other low-molecular weight alcohols can be prepared in the same way by substituting the appropriate alcohol and extending the reaction time a little.
Esters prepared by any of the following methods can be purified, if necessary, by adsorption chromatography (see below). In all the methods that follow, care should be taken in the evaporation of solvents as appreciable amounts of esters up to C14 (or even C16) can be lost if this step is performed carelessly, for example by an over vigorous use of a stream of nitrogen. Troublesome artefacts may be introduced into samples if plastic tubes or pipettes are used at any stage - always use glassware.
2. Acid-Catalysed Esterification and Transesterification
Free fatty acids are esterified and O-acyl lipids transesterified by heating them with a large excess of anhydrous methanol in the presence of an acidic catalyst. Addition of an inert solvent, such as toluene, to the reaction medium ensures that non-polar lipids dissolve and can react. Sterol esters and wax esters tend to react more slowly than other lipids.
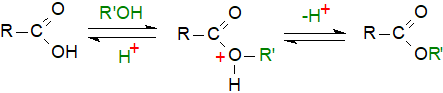 |
| Figure 1. Acid-catalysed methylation. |
One popular reagent is methanolic hydrogen chloride (NOT methanolic hydrochloric acid), and it is most easily prepared by adding acetyl chloride (5 mL) slowly to cooled dry methanol (50 mL). Methyl acetate is formed as a by-product, but it does not interfere with methylations at this concentration. A solution of 1-2% (v/v) concentrated sulfuric acid in methanol transesterifies lipids in the same manner and at much the same rate; it is very easy to prepare whenever it is required simply by carefully pipetting the appropriate amount of acid into cold methanol. Fresh reagent is best but if need be, it can be stored refrigerated for up to 4 weeks. It is usual to heat the lipid sample in the reagent under reflux for about two hours, but equally effective esterification is obtained if the reaction mixture is heated in a stoppered tube at 50°C overnight (incidentally reducing the glassware requirements). Free (unesterified) fatty acids are esterified much more rapidly (~1hr at 50°C).
| Laboratory protocol: The lipid sample (up to 5 mg) is dissolved in toluene (1 mL) in a test tube fitted with a condenser, and 1% sulfuric acid in methanol (2 mL) is added, before the mixture is refluxed for 2 hours (or alternatively the mixture can be left overnight in a stoppered tube at 50°C). Water (5 mL) containing sodium chloride (5%) is added and the required esters are extracted with isohexane (2 x 5 mL), using Pasteur pipettes to separate the layers. The isohexane layer is washed with water (4 mL) containing potassium bicarbonate (2%) and dried over anhydrous sodium sulfate. The solution is filtered to remove the drying agent, and the solvent is removed in a gentle stream of nitrogen or under reduced pressure in a rotary film evaporator. |
No solvent other than methanol is necessary if free fatty acids alone are to be methylated, or if polar lipids such as phospholipids are to be transesterified. If water is present, it may inhibit the reaction. The same method is used to prepare dimethylacetals from aliphatic aldehydes or plasmalogens (and simultaneously with methyl ester formation); their mass spectra are described here... While boron trifluoride in methanol (12-14% w/v) has been much used as a transesterification catalyst and for esterifying free fatty acids, I have many reservations about its use, and ageing reagent can cause artefact formation.
3. Base-Catalysed transesterification
O-Acyl lipids are transesterified rapidly in anhydrous methanol in the presence of a basic catalyst. Free fatty acids are not esterified, and care must be taken to exclude water from the reaction medium to prevent their formation by hydrolysis of lipids. This is the mildest procedure available and should be employed where possible when high proportions of polyunsaturated fatty acids are present in samples.
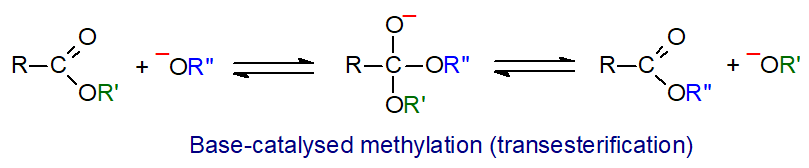 |
| Figure 2. Base-catalysed methylation. |
0.5M Sodium methoxide in anhydrous methanol, prepared simply by dissolving fresh clean sodium in dry methanol, is the most popular reagent, but potassium methoxide or hydroxide have also been used as catalysts. The reagent is stable for a month or so at room temperature if oxygen-free methanol is used in its preparation. The reaction is very rapid, so phosphoglycerides and triacylglycerols are completely transesterified in a few minutes at room temperature, but wax and cholesterol esters take longer (30 to 60 min). It is commonly performed as follows:
| Laboratory protocol: The lipid sample (up to 10 mg) is dissolved in dry toluene (1 mL) in a test-tube, 0.5M sodium methoxide in anhydrous methanol (2 mL) is added, and the solution is maintained at 50°C for 10 min. Glacial acetic acid (0.1 mL) is then added, followed by water (5 mL). The required esters are extracted into isohexane (2 x 5 mL), using a Pasteur pipette to separate the layers. The isohexane layer is dried over anhydrous sodium sulfate and filtered, before the solvent is removed in a gentle stream of nitrogen or under reduced pressure on a rotary film evaporator. |
As with acid-catalysed transesterification procedures, an additional solvent, such as toluene or tetrahydrofuran, is necessary to solubilize non-polar lipids like cholesterol esters or triacylglycerols. Aldehydes are not liberated from plasmalogens with basic reagents.
4. Clean-Up of Methyl Esters
It is sometimes necessary to purify methyl esters after transesterification has been carried out to eliminate troublesome impurities prior to analysis by gas chromatography. Cholesterol can be removed in this way from animal tissue preparations as it will elute from GC columns a few hours after the methyl esters as a broad hump on chromatograms and so may interfere with subsequent analyses. Purification is most easily accomplished by adsorption column chromatography.
| Laboratory protocol: A short column (approx. 2 cm) of silica gel or FlorisilTM in a Pasteur pipette plugged with glass wool is pre-conditioned by elution with isohexane (5 mL). Methyl esters (up to 5 mg in 0.2 mL isohexane) are added and they are recovered by elution with isohexane-diethyl ether (95:5, v/v; 10 mL), while cholesterol and other polar impurities remain on the column. Commercial solid-phase extraction columns can be used in the same way. The eluent is removed in a gentle stream of nitrogen or under reduced pressure on a rotary film evaporator. |
Methyl esters can also be purified by preparative thin-layer chromatography, with hexane-diethyl ether (9:1, v/v) as the mobile phase.
References
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis.
In: Advances in Lipid Methodology - Two, pp. 69-111 (1993) (Ed. W.W. Christie, Oily Press, Dundee) - available as a pdf file
 here....
here.... - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
| © Author: William W. Christie |  |
|
| Updated: March 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
