Mass Spectrometry of 3-Pyridylcarbinol Esters
Halogenated Fatty Acids
 As with
my other documents on mass spectrometry, this is a subjective account that details only those fatty acids relevant to this topic that
have been encountered during our research activities and for which we have spectra available for illustration purposes.
Inevitably, this has led to an uneven and incomplete treatment of the topic.
Fragmentations are discussed simplistically, as this is intended mainly as a practical as opposed to a mechanistic guide.
Spectra of 3‑pyridylcarbinol ('picolinyl') esters are described here,
but those of methyl esters
and DMOX/pyrrolidide derivatives are discussed on separate web pages.
As general features of the spectra of 3‑pyridylcarbinol esters are described in the earlier web pages in this series,
key diagnostic ions only are described.
Only a few of these spectra have been published elsewhere.
As with
my other documents on mass spectrometry, this is a subjective account that details only those fatty acids relevant to this topic that
have been encountered during our research activities and for which we have spectra available for illustration purposes.
Inevitably, this has led to an uneven and incomplete treatment of the topic.
Fragmentations are discussed simplistically, as this is intended mainly as a practical as opposed to a mechanistic guide.
Spectra of 3‑pyridylcarbinol ('picolinyl') esters are described here,
but those of methyl esters
and DMOX/pyrrolidide derivatives are discussed on separate web pages.
As general features of the spectra of 3‑pyridylcarbinol esters are described in the earlier web pages in this series,
key diagnostic ions only are described.
Only a few of these spectra have been published elsewhere.
Fluoro fatty acids were found to be perfectly stable and were easily derivatized, but chloro- and bromo-fatty acids can lose the halogen atom readily unless care is taken during derivatization.
Fluoro Fatty Acids
One natural seed oil contains several fatty acids with a fluorine atom on the terminal carbon, i.e., Dichapetalum toxicarum. We re-investigated this oil using 3‑pyridylcarbinol ester and DMOX derivatives and, in addition to obtaining novel mass spectrometric information, we found several hitherto unknown minor fatty acid components (Christie et al., 1998). We also have some mass spectra from several synthetic fluoro-fatty acids kindly donated by Dr David O'Hagan of St Andrews University with the assistance of Dr Jack Hamilton (Belfast University), and details of some of these have been published (Hamilton and Christie, 2000). They provided important evidence for some mechanistic concepts, but the aim of these pages is simply to act as a practical guide.
As might be expected, 3-pyridylcarbinol ester give informative mass spectra, which serve to locate the fluorine atom and other structural features. The mass spectrum of 3-pyridylcarbinyl 18-fluoro-octadecanoate is -
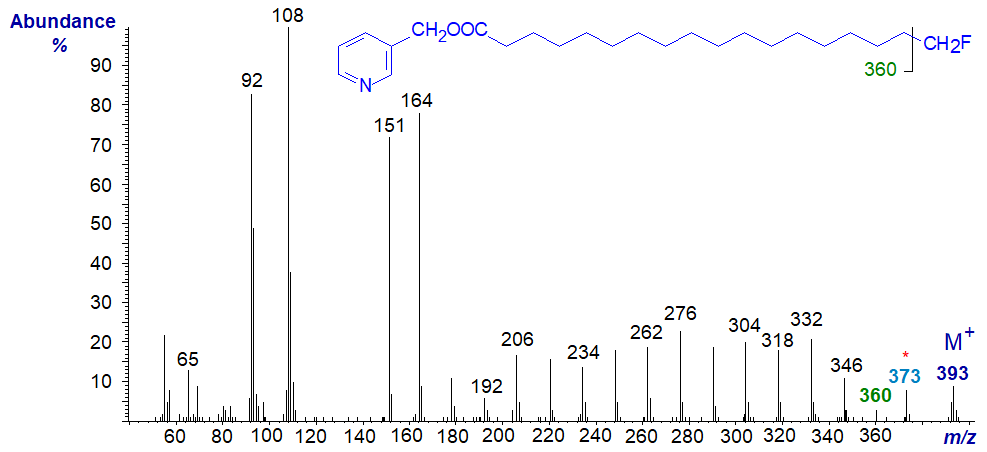
After the molecular ion, there are gaps of 20 amu to m/z = 373 (loss of HF) and one of 33 amu to 360 (loss of the terminal −CH2F). Thereafter, there are regular gaps of 14 amu for loss of successive methylene groups as in the spectrum of 3-pyridylcarbinyl octadecanoate.
Mass spectrum of 3-pyridylcarbinyl 18-fluoro-oleate -
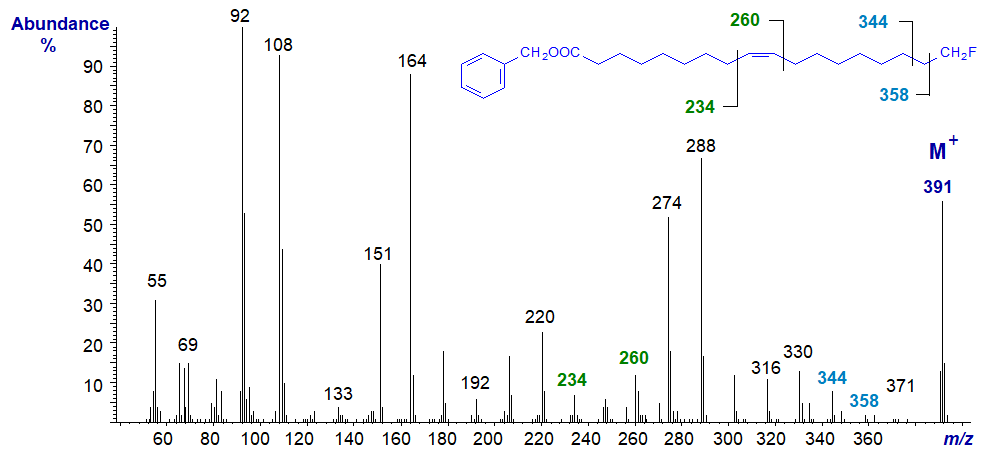
This resembles the spectrum of 3-pyridylcarbinyl oleate, and the position of the double bond is determined as described in the section of this website on 3‑pyridylcarbinol esters of monoenes. In the high mass range, there are again diagnostic gaps of 20 amu to m/z = 371 (loss of HF - just discernible) and one of 33 amu to 358 (loss of the terminal −CH2F). The ion formed by cleavage beta to the terminal carbon at m/z = 344 is a better diagnostic guide
Mass spectrum of 3-pyridylcarbinyl 18-fluoro-linoleate -
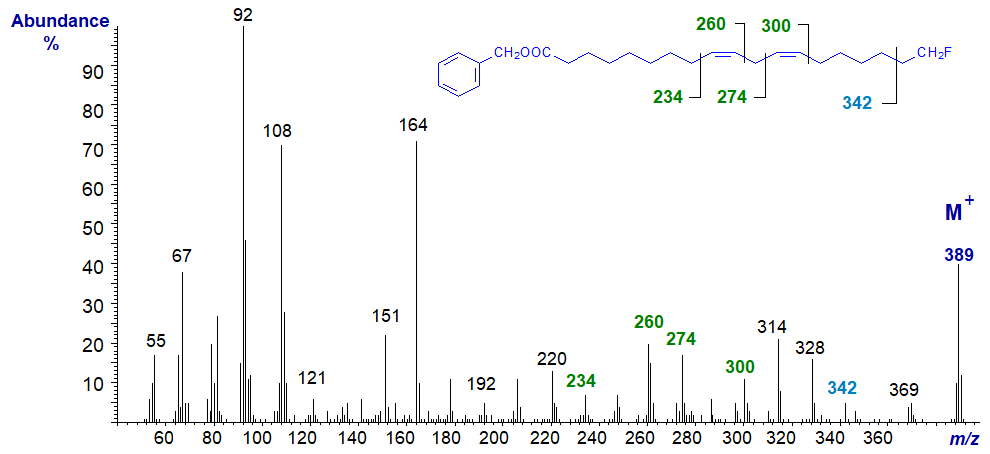
As anticipated, this is similar to the spectrum of 3-pyridylcarbinyl linoleate, and the position of the double bonds are determined as described in the section of this website on 3-pyridylcarbinol esters of dienes. In the high mass range, there is again a diagnostic gap of 20 amu to m/z = 369 (loss of HF). The expected gap of 33 amu to 356 (loss of the terminal −CH2F is not clearly seen, but the ion formed by cleavage beta to the terminal carbon at m/z = 342 is again a useful diagnostic guide.
The mass spectrum of synthetic 3-pyridylcarbinyl 2-fluoro-octadecanoate -
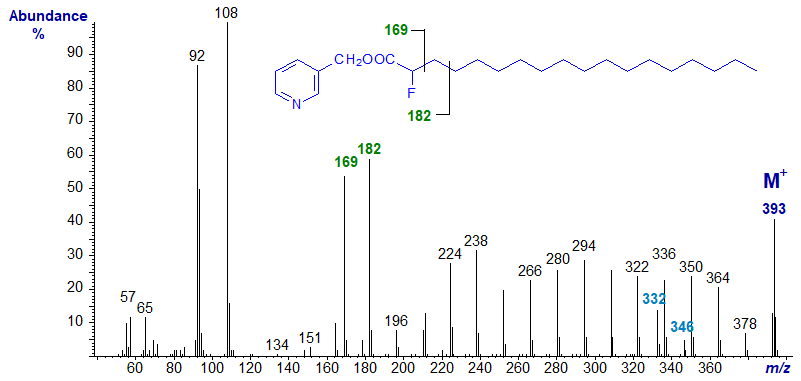
The ion normally at m/z = 151 (no longer believed to be equivalent to the McLafferty ion) is shifted to m/z = 169 as expected, and indeed all ions from here upwards are increased by 18 amu in comparison to those in the spectrum of 3-pyridylcarbinyl octadecanoate. The anomalous ion at m/z = 332 (or [M−61]+) probably represents expulsion of the three-carbon fragment from C2 to C4 and containing the fluorine atom, while that at m/z = 346 represents the loss of C2 and C3.
The mass spectrum of synthetic 3-pyridylcarbinyl 18,18,18-trifluoro-octadecanoate -
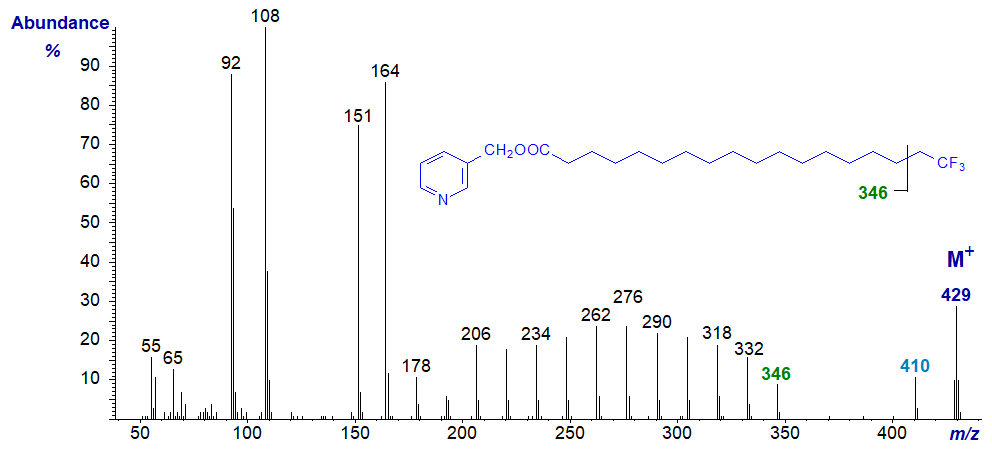
The ion at m/z = 410 presumably represents the loss of one fluorine atom from the terminal carbon, which could then undergo beta cleavage to lose an ion that could be represented by [CH2CF2]+ to give the ion at m/z = 346. Alternatively, it may simply be a separate beta fragmentation with loss of [CH2CF3]+.
Chloro Fatty Acids
`We only have spectra available of 3-pyridylcarbinol esters of a few omega-chloro fatty acids, produced by microbial fermentation (Rhodococcus rhodocrous) of chlorohydrocarbons by Jack Hamilton (Belfast). Other chlorinated fatty acids have been found in marine organisms, but we do not have access to these. The 3‑pyridylcarbinol esters here were prepared by hydrolysing to the free acid followed by the mild derivatization procedure of Balazy and Nies (1989).
The mass spectrum of 3-pyridylcarbinyl 16-chloro-hexadecanoate -
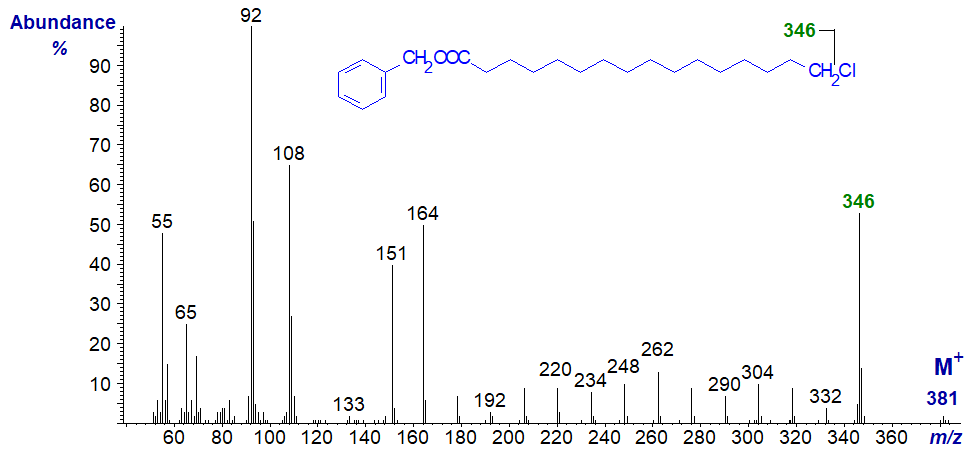
Following the molecular ion at m/z = 381, there is a gap of 35 amu to m/z = 346 for loss of the terminal chlorine atom. Thereafter, there is a regular series of ions 14 amu apart for cleavage at successive methylene groups.
Mass spectrum of 3-pyridylcarbinyl 16-chloro-hexadec-9-enoate -
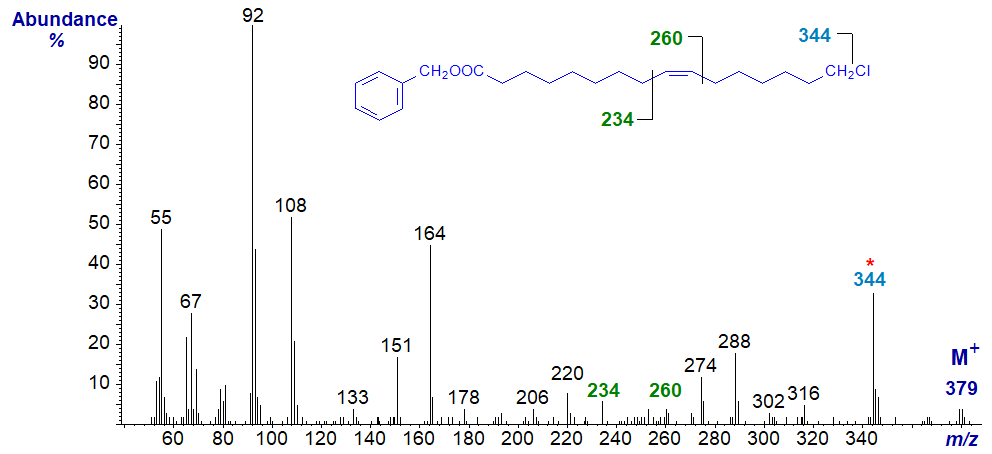
Again, there is a gap of 35 amu to m/z = 344 for loss of the terminal chlorine atom. Thereafter, there is at first a regular series of ions 14 amu apart for cleavage at successive methylene groups until the double bond is reached, the position of which is determined as described in the section of this website on 3-pyridylcarbinol esters of monoenes.
Bromo Fatty Acids
Brominated fatty acids have been found in many marine organisms, but we do not have access to these. Although our first attempts to prepare derivatives of bromo fatty acids (produced by microbial fermentation of bromohydrocarbons) failed when derivatization procedures caused elimination of bromine with formation of ethoxy or methoxy fatty acids (see the section of this website on pyridylcarbinol esters of epoxy, furanoid and alkoxy fatty acids), synthetic 2-bromo-octadecanoic acid from a commercial source was successfully derivatized by the method of Balazy and Nies (1989). 3-Pyridylcarbinyl 2-bromo-hexadecanoate has the rather uninteresting mass spectrum -
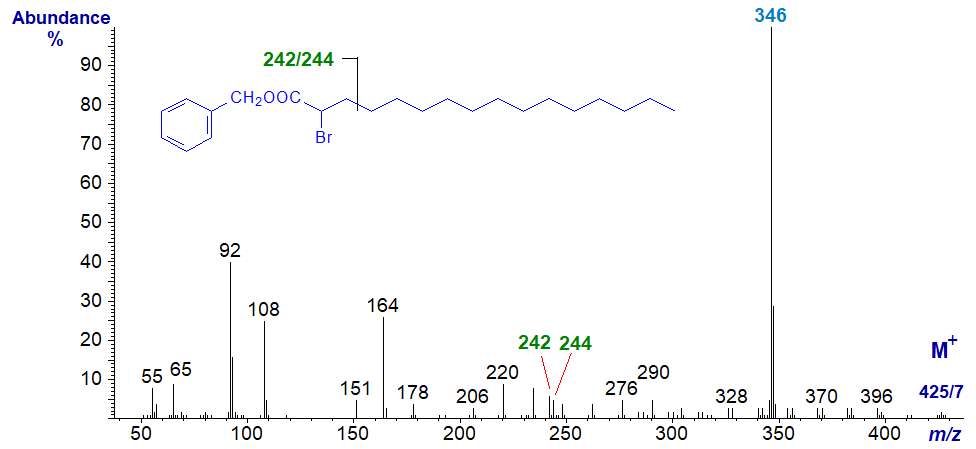
The small molecular ion is a doublet (m/z = 425/7), but the base ion at m/z = 346 reflects loss of a bromine atom (as a reminder, bromine exists as two isotopes in approximately equal amounts of atomic mass 79 and 81). The doublet of ions at m/z = 242/244 presumably results from cleavage between carbons 3 and 4, and there are a few small ions in the higher mass region that are doublets so presumably contain bromine.
Further mass spectra of halogenated fatty acids in the form of the various derivatives are available, but without interpretation, in the Archive Sections of these web pages.
References
- Balazy, M. and Nies, A.S. Characterization of epoxides of polyunsaturated fatty acids by mass spectrometry via 3-pyridinylmethyl esters. Biomed. Environ. Mass Spectrom., 18, 328-336 (1989); DOI.
- Christie, W.W., Hamilton, J.T.G. and Harper, D.B. Mass spectrometry of fluorinated fatty acids in the seed oil of Dichapetalum toxicarium. Chem. Phys. Lipids, 97, 41-47 (1998); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
| © Author: William W. Christie |  |
|
| Updated: November 22nd, 2023 | Contact/credits/disclaimer | |
