Mass Spectrometry of 3-Pyridylcarbinol Esters
Epoxy, Furanoid and Alkoxy Fatty Acids
 As with
my other documents on mass spectrometry, this is a subjective account that details only those relevant fatty acids encountered
during our research activities and for which we have spectra available for illustration purposes.
Inevitably, this has led to an uneven and incomplete treatment of the topic.
Fragmentations are discussed simplistically as this is intended mainly as a practical guide, but earlier documents in this series,
especially that dealing with these derivatives of saturated fatty acids,
discuss some basic mechanistic concepts.
3‑Pyridylcarbinol ('picolinyl') esters, DMOX derivatives and pyrrolidides usually permit location
of ring structures and other structural features including double bonds.
One may be better than another for a specific type of fatty acid, but in this instance, I have insufficient data to state a preference.
My general philosophy is to always to prepare more than one derivative when possible.
Only a few of these spectra have been published elsewhere to our knowledge, but prior publications are cited when known.
Mass spectra of methyl esters
and of DMOX and pyrrolidine derivatives are described in separate documents.
There is an account of the natural occurrence of epoxy and furanoid fatty acids in the
Lipid Essentials section.
As with
my other documents on mass spectrometry, this is a subjective account that details only those relevant fatty acids encountered
during our research activities and for which we have spectra available for illustration purposes.
Inevitably, this has led to an uneven and incomplete treatment of the topic.
Fragmentations are discussed simplistically as this is intended mainly as a practical guide, but earlier documents in this series,
especially that dealing with these derivatives of saturated fatty acids,
discuss some basic mechanistic concepts.
3‑Pyridylcarbinol ('picolinyl') esters, DMOX derivatives and pyrrolidides usually permit location
of ring structures and other structural features including double bonds.
One may be better than another for a specific type of fatty acid, but in this instance, I have insufficient data to state a preference.
My general philosophy is to always to prepare more than one derivative when possible.
Only a few of these spectra have been published elsewhere to our knowledge, but prior publications are cited when known.
Mass spectra of methyl esters
and of DMOX and pyrrolidine derivatives are described in separate documents.
There is an account of the natural occurrence of epoxy and furanoid fatty acids in the
Lipid Essentials section.
Epoxy Fatty Acids
Epoxy fatty acids occur naturally in several seed oils. Mass spectra of 3-pyridylcarbinol esters of epoxy fatty acids were first described by Balazy and Nies (1989). Note that special methods are required for successful preparation of the derivative because of the sensitivity of the epoxyl moiety to acidic conditions. The mass spectrum of synthetic 3-pyridylcarbinyl 9,10-epoxy-octadecanoate is -
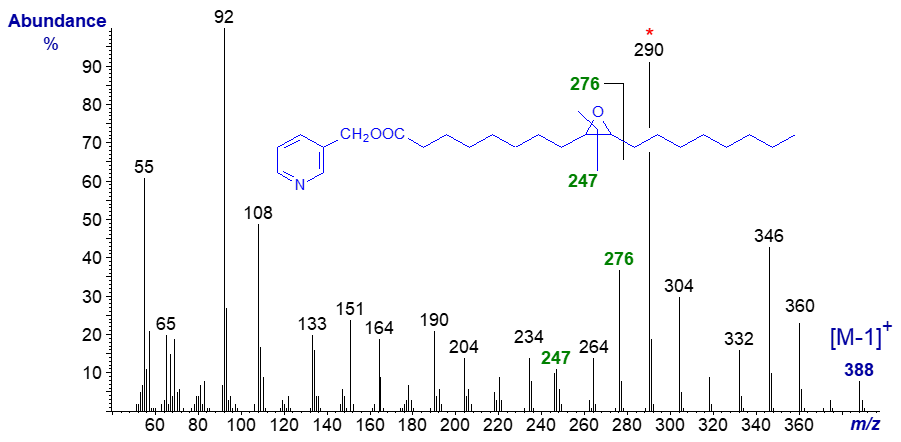
The ions at m/z = 92, 108, 151 and 164 are characteristic of the 3-pyridylcarbinol moiety (see our web page on the analogous saturated derivatives). The ring structure is between the ions at m/z = 234 and 276, but that at m/z = 247 (unusual in being odd-numbered) is an invaluable diagnostic guide. Interestingly, the last ion is present and is of definitive value in the spectra of analogous cyclopropanoid fatty acids (see the section of this website on mass spectra of cyclic fatty acids), while the ion at m/z = 290 formed by cleavage beta to the ring is distinctive.
The mass spectrum of 3-pyridylcarbinyl 12,13-epoxy-octadec-9-enoate or vernolate (first illustrated by Balazy and Nies (1989)), which was the first natural epoxy fatty acid to be characterized (by the eminent lipid chemist F.D. Gunstone) follows; the sample was from the seed oil of Vernonia galamensis.
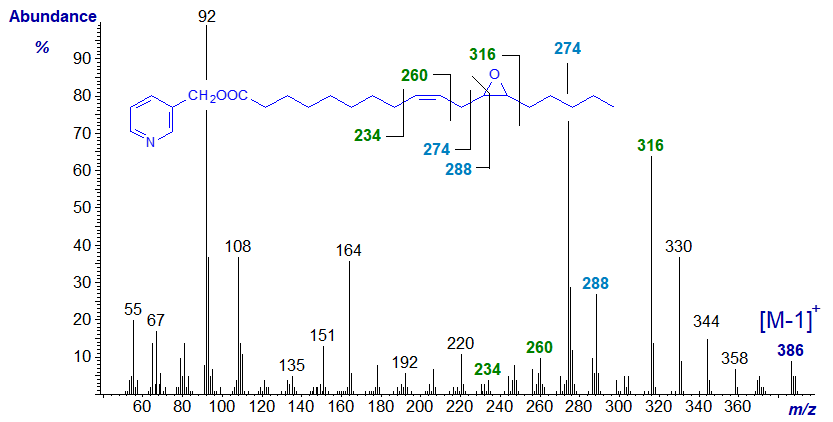
The gap of 26 amu between m/z = 234 and 260, and of 40 amu between m/z = 220 and 260, serve to locate the double bond in position 9, while the two substantial ions at m/z = 274 and 288 are akin to those in 3‑pyridylcarbinol esters of 9‑monoenes. The first of these is formed by cleavage alpha to the ring and beta to the double bond, influences that may explain why it is so abundant. In this instance, the ion at m/z = 288 is part of the ring structure, and that at m/z = 316 represents cleavage on the distal side of the ring.
Furanoid Fatty Acids
Unfortunately, we do not have available mass spectra of the furanoid fatty acids with methyl substituents on the ring, which occur naturally in small amounts in plant lipids and via the food chain in fish. Furanoid fatty acids can be formed by autoxidation of conjugated fatty acids, as with the spectrum illustrated (from Matreya Inc., USA). The 3-pyridylcarbinol ester derivative of 8-(5-hexyl-2-furanyl)-octanoate (spectrum not published elsewhere) -
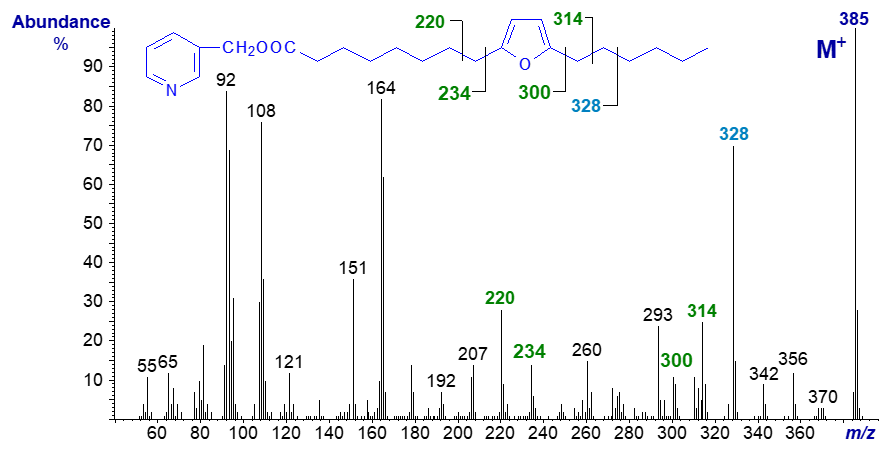
As is usual with 3-pyridylcarbinol esters, there is a distinctive fingerprint spectrum, but interpretation is not as straightforward as I have come to expect with such derivatives. A gap of 66 amu between m/z = 234 and 300 for cleavage on either side of the ring might have been expected, but this is hardly obvious. On the other hand, the ions formed by cleavage beta to the ring (m/z = 220 and 314) are more distinct, as with other derivatives of this acid. I presume that the ring must open under electron bombardment so that a series of alternative fragmentations occurs, for example to give the ion at m/z = 293. The large ion at m/z = 328 is formed by cleavage between carbons 14 and 15, possibly with formation of a stable conjugated system via formation of a third double bond. Perhaps surprisingly, methyl esters of furanoid fatty acids are arguably the best derivatives for the common range encountered in fish oils.
Ethoxy and Methoxy Fatty Acids
Although methoxy fatty acids are present many organisms often of marine origin, those whose spectra are illustrated here were formed as artefacts while attempting to hydrolyse or methylate brominated fatty acids produced from brominated hydrocarbons by microorganisms (with Dr Jack Hamilton of Queens University, Belfast). None of these spectra have been published elsewhere.
3-Pyridylcarbinol 15-ethoxy-pentadecanoate -
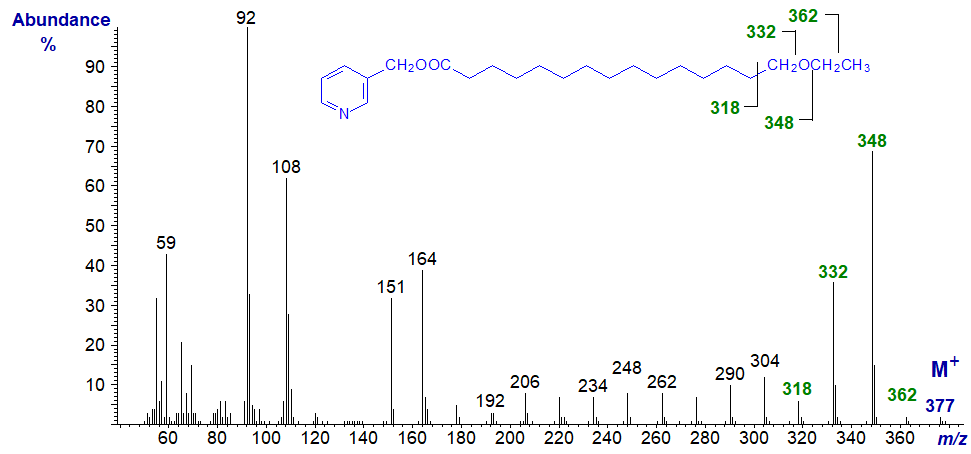
Interpretation of the spectrum of this saturated fatty derivative with a terminal ethoxy group could not be more straight forward and indeed is analogous to that of a conventional saturated analogue. Starting with the molecular ion at m/z = 377, we lose the terminal methyl group to form m/z = 362, the adjacent methylene group, i.e., a gap of 14 amu to m/z = 348, followed then by a gap of 16 amu for loss of the oxygen atom to m/z = 332. The remaining ions are spaced uniformly 14 amu apart for loss of successive methylene groups.
We have the spectrum of a related fatty acid with a methyl group in a central location, i.e., 3-pyridylcarbinyl 10-methyl,17-ethoxy-heptadecanoate in our Archive section. The methyl group is easily located, but I will allow readers to work it out.
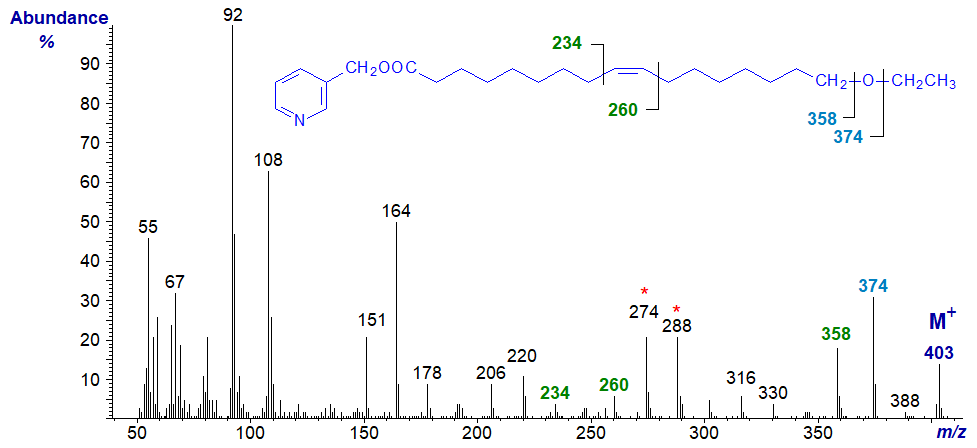
In the spectrum of 3-pyridylcarbinyl 17-ethoxy-heptadec-9-enoate, the double bond in position 9 is easily recognized by the gap of 26 amu between m/z = 234 and 260 and the doublet of ions at m/z = 274 and 288, as discussed above for the spectrum of the derivative of vernolic acid. Similarly, the terminal ethoxyl group is easily characterized by the gap of 29 amu for the loss of the terminal ethyl moiety, between the molecular ion (m/z = 403) and that at m/z = 374; a further gap of 16 amu to m/z = 358 represents the loss of the oxygen atom.
The mass spectrum of an alkoxy fatty acid derivative with a methoxyl group on the penultimate carbon atom is very different, as for 3‑pyridylcarbinol 13-methoxy-tetradecanoate illustrated next.
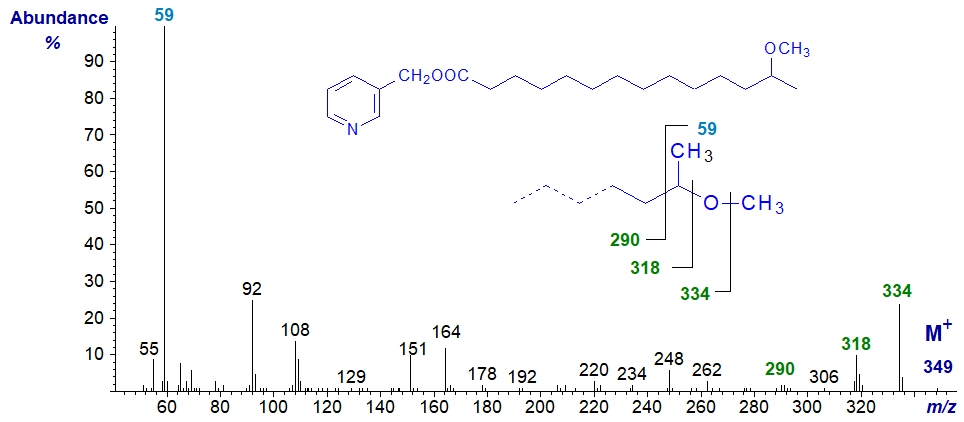
It is best considered as a fatty acid with a terminal methoxy group adjacent to a methyl branch as depicted on the inset formula in the spectrum. Rather than being one of the pyridine-containing ions, the base ion is unusually at m/z = 59 and dominates the spectrum. Presumably this unique feature represents a fragment from the terminal part of the molecule containing the last two carbon atoms and the attached methoxyl group. The ion at m/z = 334 is from loss of the methyl group attached to oxygen, not the terminal carbon atom in the chain, while the next ion 16 amu down at m/z = 318 is for loss of the oxygen atom.
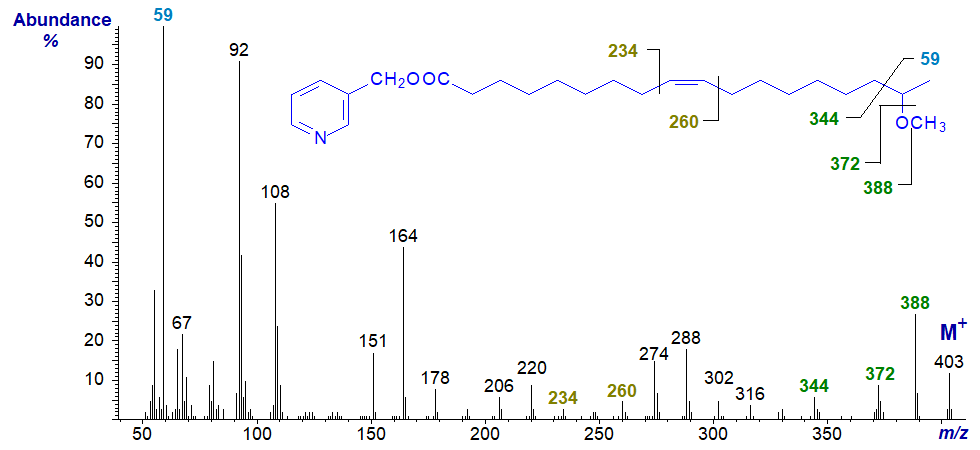
In the spectrum of an unsaturated analogue 3-pyridylcarbinyl 17-methoxy-octadec-9-enoate, the molecular ion is more abundant, but the base ion is again at m/z = 59. The fragmentations around the methoxyl group are as in the previous spectrum. In this instance, the double bond is located by the gap of 26 amu between m/z = 234 and 260 and the doublet of ions at m/z = 274 and 288 as in an earlier spectrum in this section.
References
- Balazy, M. and Nies, A.S. Characterization of epoxides of polyunsaturated fatty acids by mass spectrometry via 3-pyridinylmethyl esters. Biomed. Environ. Mass Spectrom., 18, 328-336 (1989); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: November 22nd, 2023 | Contact/credits/disclaimer | |
