Mass Spectrometry of Methyl Esters
Thia Fatty Acids
As with my other documents on mass spectrometry, this is a subjective account that details only those relevant fatty acids encountered during my research activities and for which we have spectra available for illustration purposes. The spectra described here are of synthetic fatty acids, prepared by my friend and collaborator Professor Marcel Lie Ken Jie of Hong Kong University, and they do not occur in nature. However, these and related hetero-atom fatty acids are being considered for therapeutic purposes and have biochemical applications. Their mass spectra may be of interest from the standpoint of mechanistic mass spectrometry, but this is not my primary concern here.
Mass spectra of methyl esters are described in this document, but I describe key diagnostic ions only, as general features of the spectra of these derivatives are described in the other web pages in this section. I have no spectra for DMOX or pyrrolidine derivatives, as I was not using these during my work at the time, but the spectra of 3‑pyridylcarbinol derivatives are described in a separate document. Details of the spectra were first published in -
Christie, W.W., Lie Ken Jie, M.S.F., Brechany, E.Y. and Bakare, O. Mass spectral characterization of 3-picolinyl and methyl ester derivatives of isomeric thia fatty acids. Biomed. Environm. Mass Spectrom., 20, 629-635 (1991); DOI.
In this web page, I describe spectra of mono-thia-stearates only for illustrative purposes. Information on a series of dithia-stearates is available in the above paper, but the details are only likely to be of interest to a few specialists, so they are not discussed here.
 Methyl
esters of thia stearates give distinctive spectra from which the position of the sulfur atom is easily deduced.
With all the isomers, cleavage occurs on either side of the sulfur atom to give two principal fragments in each instance as illustrated.
The relative proportions of these ions vary from isomer to isomer, but in general the ions
A1 and A2 are most abundant, while an ion representing loss of methanol from
A1 is diagnostic when the sulfur atom is remote from the carboxyl group.
In a high proportion of the isomers, either ion A1 or A2 is the base ion.
If ion B1 loses the elements of methanol, it will give an ion numerically identical to ion A1
in low-resolution mass spectrometry.
Ion B2 is usually small and can be ignored for all practical purposes.
Methyl
esters of thia stearates give distinctive spectra from which the position of the sulfur atom is easily deduced.
With all the isomers, cleavage occurs on either side of the sulfur atom to give two principal fragments in each instance as illustrated.
The relative proportions of these ions vary from isomer to isomer, but in general the ions
A1 and A2 are most abundant, while an ion representing loss of methanol from
A1 is diagnostic when the sulfur atom is remote from the carboxyl group.
In a high proportion of the isomers, either ion A1 or A2 is the base ion.
If ion B1 loses the elements of methanol, it will give an ion numerically identical to ion A1
in low-resolution mass spectrometry.
Ion B2 is usually small and can be ignored for all practical purposes.
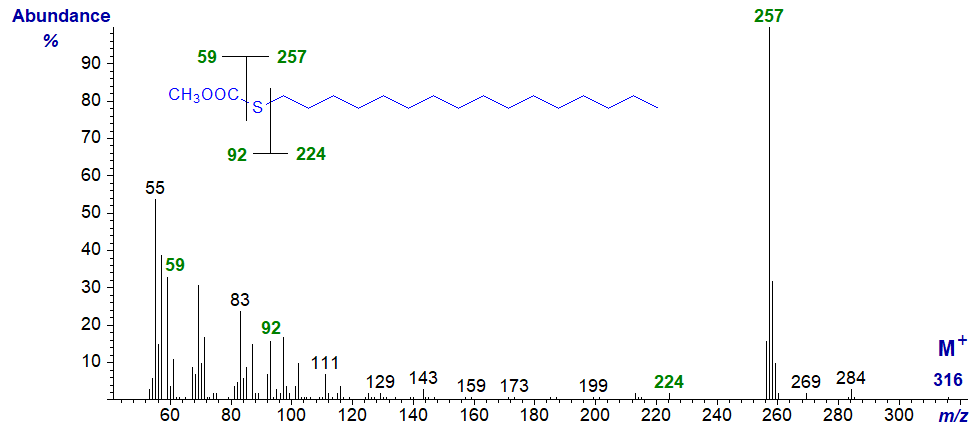
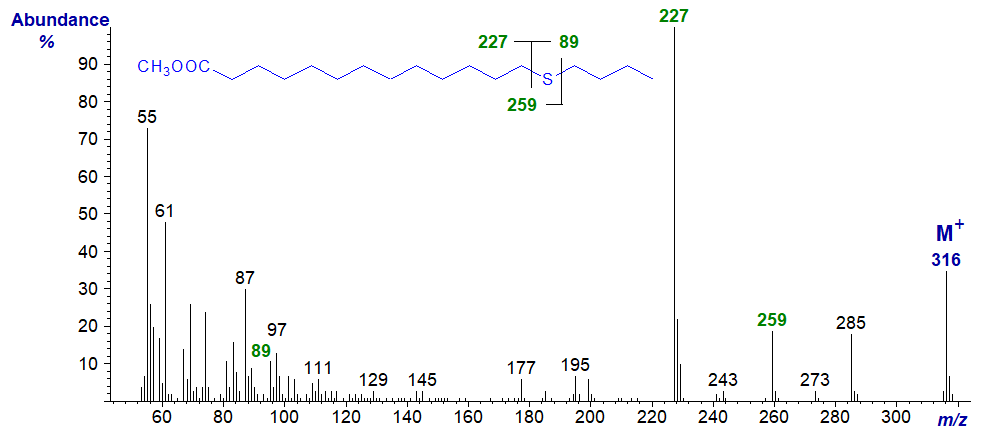
Only with methyl 2-thia stearate is it hard to locate the molecular ion (m/z = 316), and in this instance some other potentially useful ions, for example that for loss of methanol ([M−32]+) together with the McLafferty ion (at m/z = 92 for this isomer) are not very abundant. The McLafferty ion in its usual position at m/z = 74 is present in most of the remaining isomers, except for those with the sulfur atom in positions 4 and 6. With the first of these, the absence of a hydrogen atom on C4 explains the loss (see or web page on mass spectrometry of methyl esters of saturated fatty acids for a more detailed explanation). With the 6S isomer, the McLafferty ion appears to be at m/z = 73 (and this is also true for the spectrum of 6-thia-laurate). Data for the main diagnostic ions are listed in Table 1.
Table 1. Relative abundances of ions for fragmentations adjacent to the sulfur atom in mass spectra of methyl esters (see figure). |
||||
| Positional isomer | Ions (%) | |||
|---|---|---|---|---|
| A1 | A2 | B1 | B2 | |
| 2 | 59 (38) | 257 (100) | 92 (8)* | 224 (2) |
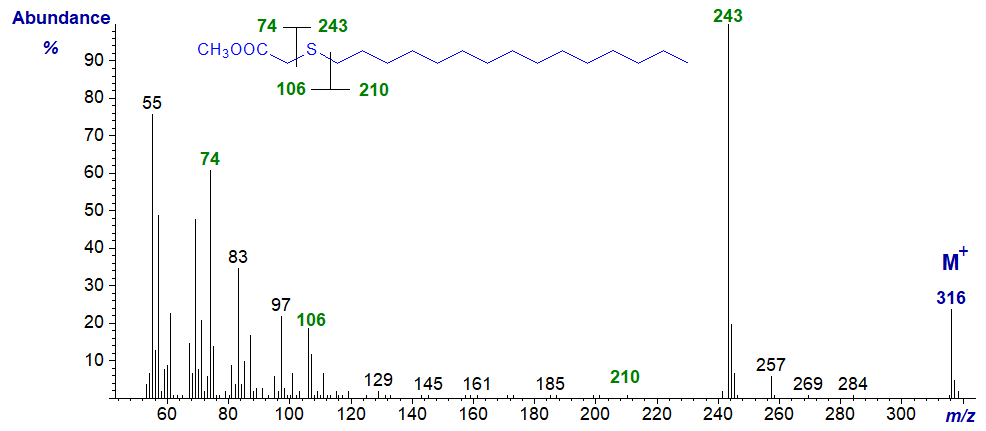
| 3 | 73 (6) | 243 (100) | 106 (19)* | 210 (1) |
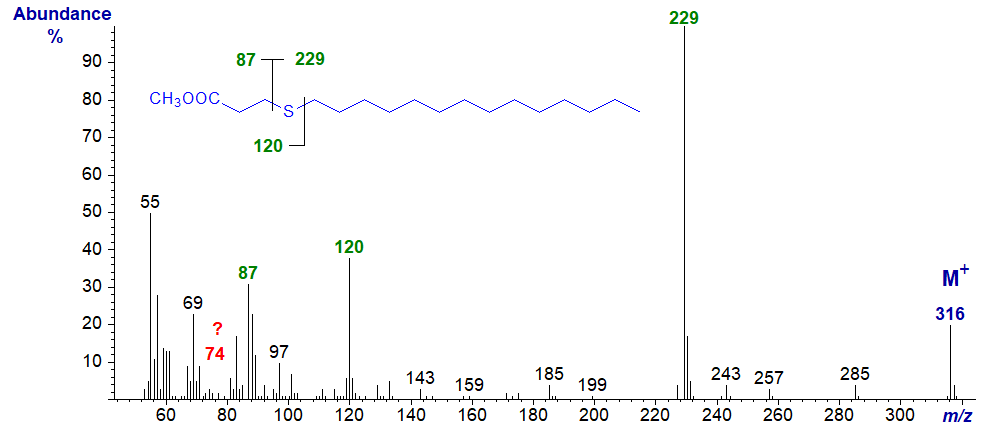
| 4 | 87 (32) | 229 (100) | 120 (39)* | - |
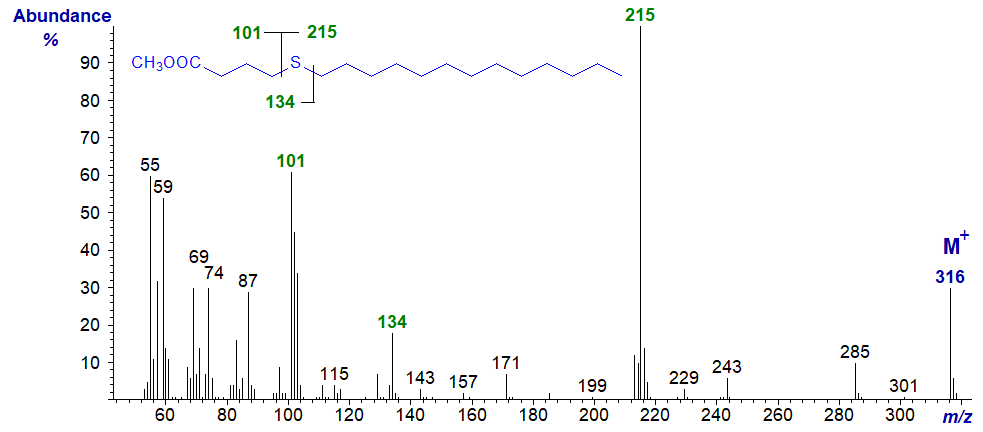
| 5 | 101 (61) | 215 (100) | 134 (18)* | - |
| 6 | 115 (100) | 201 (43) | 147 (3) | - |
| 7 | 129 (100) | 187 (64) | 161 (9) | - |
| 8 | 143 (100) | 173 (78) | 175 (19) | 141 (4) |
| 9 | 157 (81) | 159 (76) | 189 (18) | 127 (1) |
| 10 | 171 (96) | 145 (86) | 203 (21) | 113 (2) |
| 11 | 185 (72) | 131 (63) | 217 (16) |
99 (2) |
| 12 | 199 (94) | 117 (52) | 231(14) | 85 (6) |
| 13 | 213 (100) | 103 (43) | 245 (19) | 71 (10) |
| 14 | 227 (100) | 89 (9) | 259 (19) | 57 (21) |
| 15 | 241 (100) | 75 (6) | 273 (17) | 43 (53) |
| 16 | 255 (100) | 61 (14) | 287 (35) | |
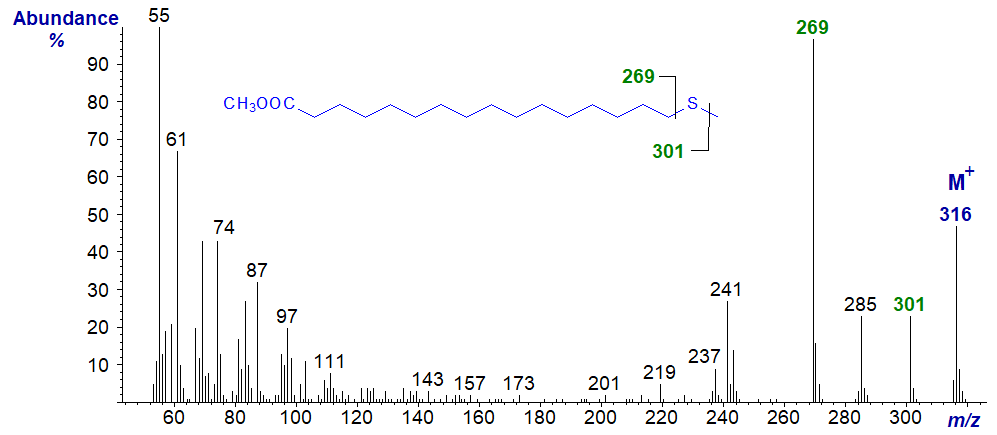
| 17 | 269 (97) | 47 (8) | 301 (23) | |
| * at B1 +1 with this isomer | ||||
The mass spectra of the selected isomers that follow are offered without further comment as the main fragments are depicted on the figures.
Methyl 2-thia-stearate
Methyl 3-thia-stearate
Methyl 4-thia-stearate
Methyl 5-thia-stearate
Methyl 6-thia-stearate
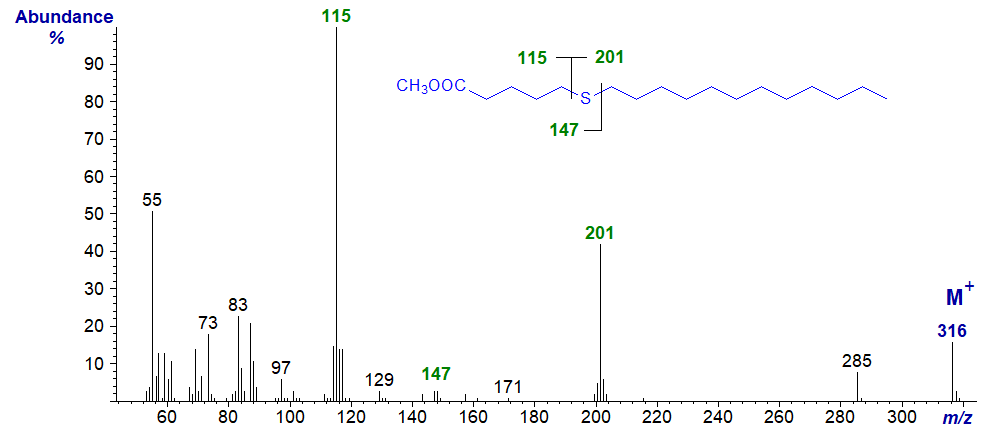
Methyl 9-thia-stearate
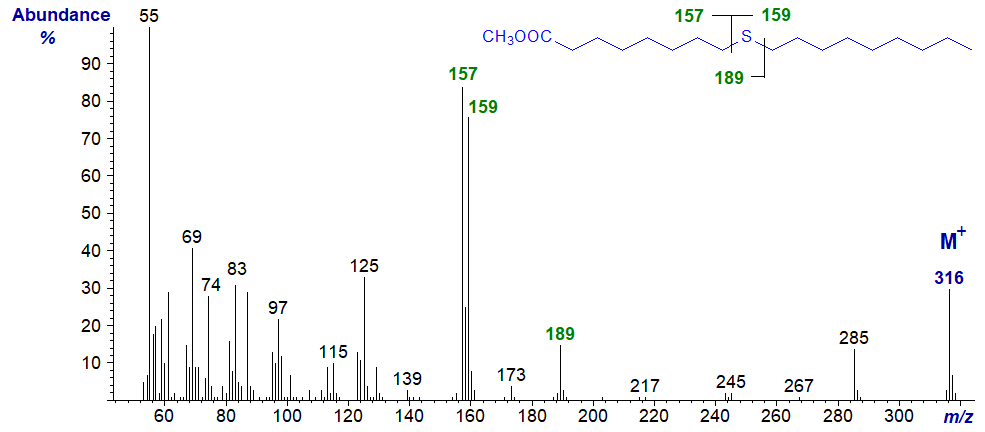
Methyl 11-thia-stearate
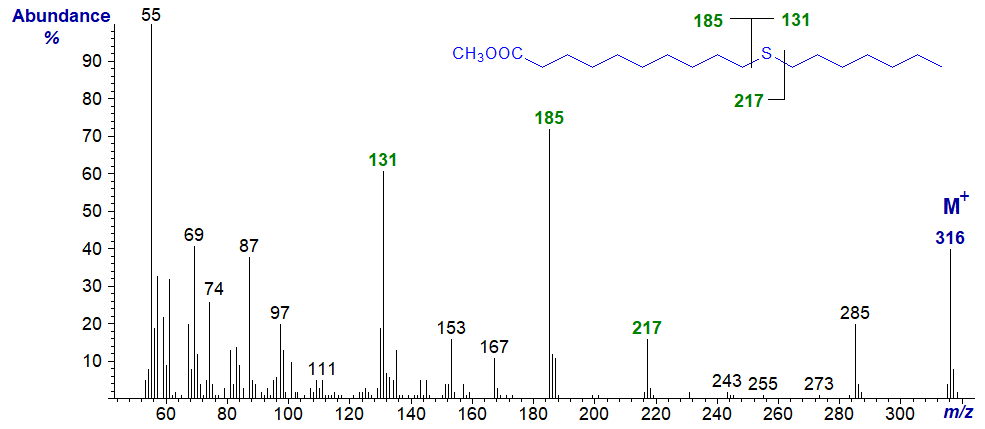
Methyl 13-thia-stearate
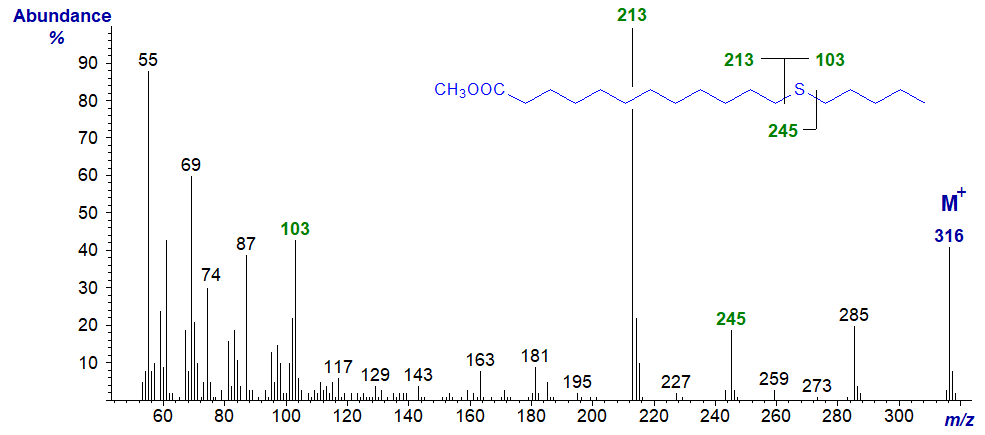
Methyl 14-thia-stearate
Methyl 17-thia-stearate
Mass spectra not illustrated, including the methyl esters of the intermediate isomers, 6-thia-laurate, and a series of dithia-stearates, are available in our mass spectral Archive web page but without interpretation.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
