Mass Spectrometry of Dimethyloxazoline and Pyrrolidine Derivatives
Epoxy, Furanoid and Alkoxy Fatty Acids
 As with my
other documents on mass spectrometry, this is a subjective account that details only those relevant
fatty acids that have been encountered during our research activities and for which we have spectra available for illustration purposes.
My apologies if this leads to an uneven treatment.
3-Pyridylcarbinol ('picolinyl') esters, DMOX derivatives and pyrrolidides usually permit location of ring structures
and other structural features including double bonds.
One may be better than another for a specific class, but my general philosophy is to always to prepare more than one derivative when possible.
Only a few of the following spectra have been published elsewhere to our knowledge, but prior publications are cited when known.
As this is intended as a practical guide only, I show mass spectrometric fragmentations in an overly simplified manner,
though earlier documents in this series, especially those dealing with derivatives of saturated fatty acids,
document some basic mechanistic concepts.
Mass spectra of methyl esters
and of 3‑pyridylcarbinol esters are described in separate documents.
There is an account of the natural occurrence of oxygenated fatty acids such as these in the
Lipid Essentials section.
As with my
other documents on mass spectrometry, this is a subjective account that details only those relevant
fatty acids that have been encountered during our research activities and for which we have spectra available for illustration purposes.
My apologies if this leads to an uneven treatment.
3-Pyridylcarbinol ('picolinyl') esters, DMOX derivatives and pyrrolidides usually permit location of ring structures
and other structural features including double bonds.
One may be better than another for a specific class, but my general philosophy is to always to prepare more than one derivative when possible.
Only a few of the following spectra have been published elsewhere to our knowledge, but prior publications are cited when known.
As this is intended as a practical guide only, I show mass spectrometric fragmentations in an overly simplified manner,
though earlier documents in this series, especially those dealing with derivatives of saturated fatty acids,
document some basic mechanistic concepts.
Mass spectra of methyl esters
and of 3‑pyridylcarbinol esters are described in separate documents.
There is an account of the natural occurrence of oxygenated fatty acids such as these in the
Lipid Essentials section.
Despite having very different structures, dimethyloxazoline and pyrrolidide derivatives of a given fatty acid have identical molecular weights and usually fragment in a similar way in the mass spectrometer under electron-impact ionization. For this reason, it is convenient to discuss both together here. DMOX derivatives tend to give more abundant diagnostic ions and have better gas chromatography properties, but pyrrolidides are better when the functional group is in a terminal position. Ions at m/z = 113 and 126 from the carboxyl group and the derivatizing moiety are usually prominent in both.
Epoxy Fatty Acids
Mass spectra in the form of one or other of the derivatives of two of the epoxy fatty acids that occur naturally in certain seed oils are described below. Note that care must be taken in preparing the derivatives for mass spectrometry, as strong acids can cause ring opening.
Spectra of DMOX derivatives of epoxy fatty acids have been described elsewhere (Marx and Classen, 1994). The mass spectrum of the DMOX derivative of 12,13‑epoxy-octadec-9-enoate or vernolate is -
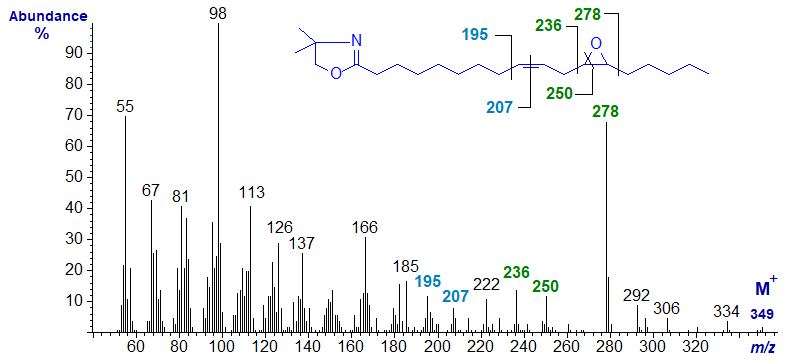
The ions at m/z = 236 and 278 serve to locate the oxirane ring (that at m/z = 250 is also relevant). The gap of 12 amu for the double bond in position 9 is between m/z = 195 and 207 (not 196 and 208 as might have been expected for DMOX derivatives of monoenes).
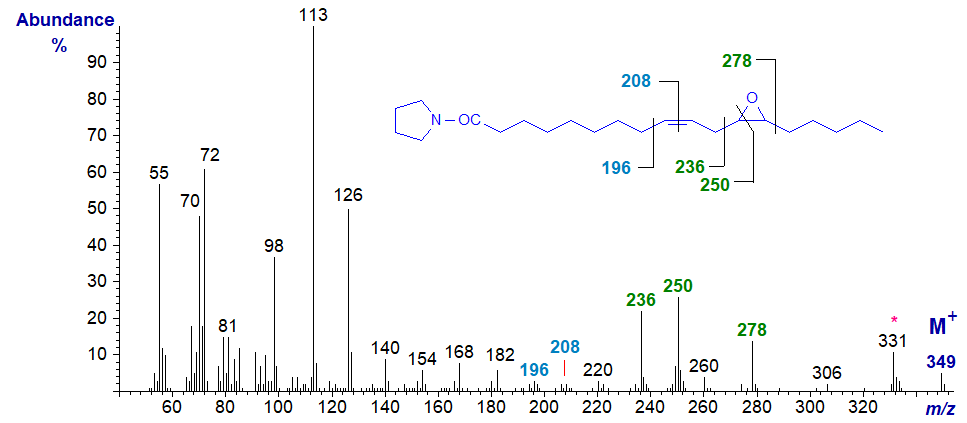
The pyrrolidine derivative of vernolic acid is much easier to prepare by starting from the methyl ester. As expected, its mass spectrum resembles that of the DMOX derivative, but some of the diagnostic ions are more clearly seen. For example, the gap of 12 amu between m/z = 196 and 208 serves to locate the double bond in position 9, while the ring structure is defined by the gap of 42 amu between m/z = 236 and 278, with the ion at m/z = 250 reflecting cleavage after carbon 12. It is perhaps surprising that the next significant ion following the molecular ion at m/z = 331 represents the loss of the elements of water.
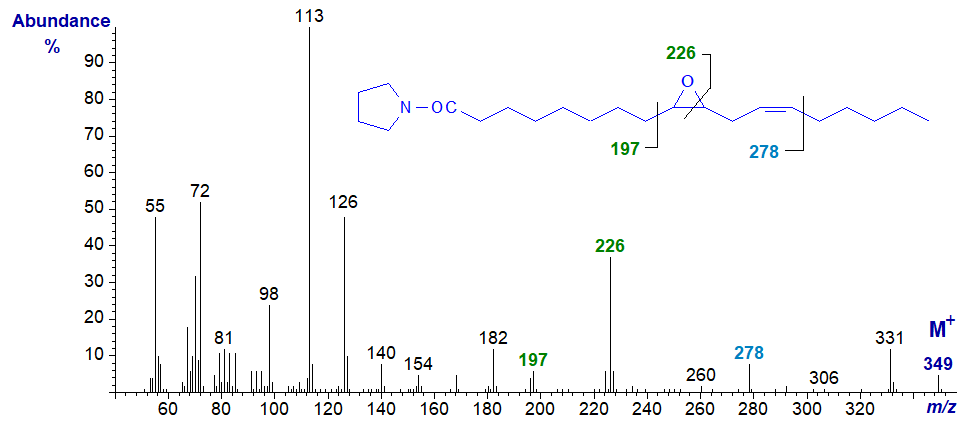
The pyrrolidine derivative of 9,10-epoxy-octadec-12-enoate or coronarate from Xeranthemum annuum seed oil has a mass spectrum with some unexpected features, as the mode of fragmentation is different from that of vernolate. There appear to be ions that locate the epoxy ring as illustrated below, but the double bond is located only indirectly by the ion at m/z = 278.
Furanoid Fatty Acids
Furanoid fatty acids with methyl substituents on the ring occur naturally in small amounts in plant lipids including algae and certain seed oils, and via the food chain, they are present in small amounts in animal tissues and especially those of fish. The simplest furanoid fatty acid, 8-(5-hexyl-2-furanyl)-octanoic or 9,12-epoxy-octadeca-9,11-dienoic acid, has been found in plants and in fish, but it can be formed on oxidation of conjugated dienoic acids, e.g., 9-cis,11-trans-octadecadienoic acid and isomers. Perhaps surprisingly, methyl esters give informative mass spectra with furanoid fatty acids. The mass spectrum of the DMOX derivative of 8-(5-hexyl-2-furanyl)-octanoate (standard purchased from Matreya Inc., U.S.A.) is -
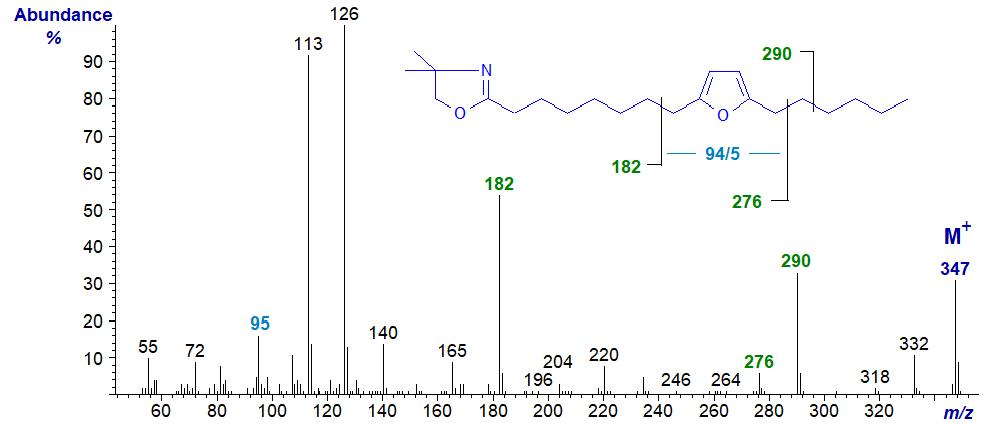
In this instance, interpretation is relatively straightforward, if somewhat unexpected in view of what is known of fragmentation mechanisms of DMOX derivatives. The ions for cleavage immediately adjacent to either side of the ring are not apparent, but those at m/z = 182 and 276 are formed by cleavage beta to it on each side. The ion at m/z = 95 is characteristic for this ring structure.
Mass spectra of pyrrolidide and DMOX derivatives of natural furanoid acids with methyl branches have useful mass spectra, and although they do not appear to have been illustrated formally elsewhere, Imbs et al. (2009) have discussed the main features of spectra of pyrrolidine derivatives of such acids. The mass spectrum of the pyrrolidide of 10,13-epoxy-11-methyl-octadecadienoate is -
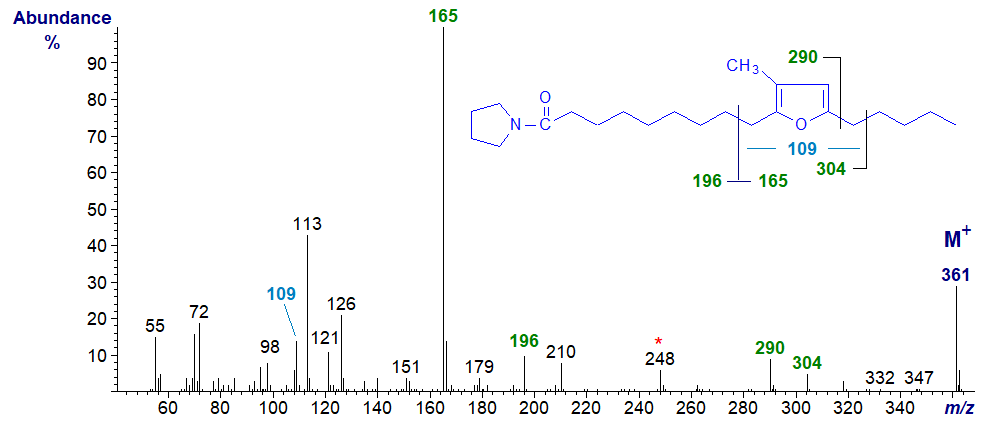
Unusually for a nitrogen-containing derivative, the base ion is not the McLafferty ion at m/z = 113, but the fragment from the terminal region of the molecule at m/z = 165, as in the spectrum of the methyl ester derivative. The ion at m/z = 109 is presumably the same as that in the spectrum of the methyl ester and confirms that there is only one methyl group in the ring. On the other hand, there are indeed ions that contain the carboxy-pyrrolidine component and locate the furanoid ring structure as marked on the spectrum. In addition, there is an intriguing ion at m/z = 248, which could be interpreted as a fragment containing carbons 10 and 11, the latter with its associated methyl group. Spectra of more isomers would be required to confirm this, but it might be a useful method for locating ring methyl groups in unknowns.
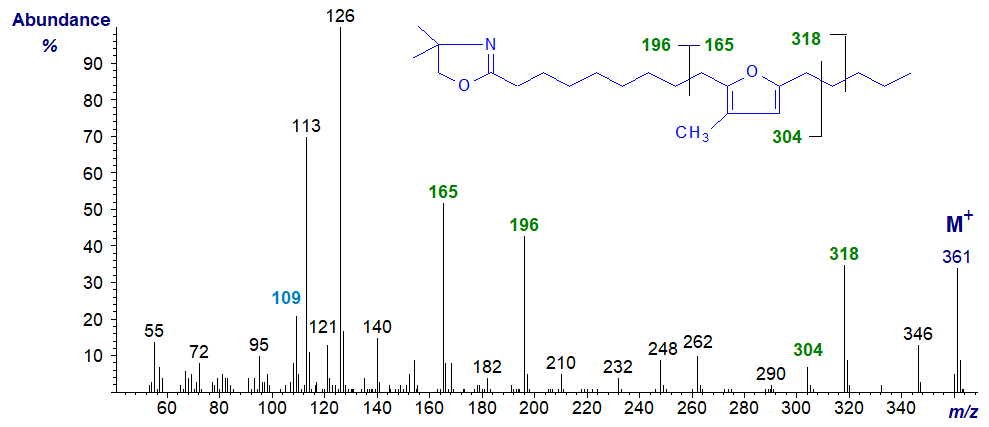
Essentially the same features are seen in the spectrum of the DMOX derivative of 10,13-epoxy-11-methyl-octadecadienoate, except that ions for cleavage beta to the ring and containing the carboxyl moiety are somewhat different in intensity (that at m/z = 165 is no longer the base ion).
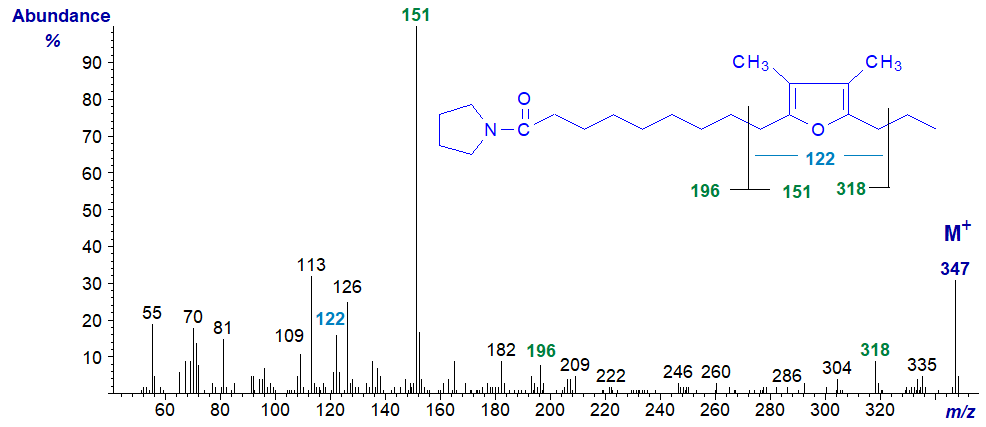
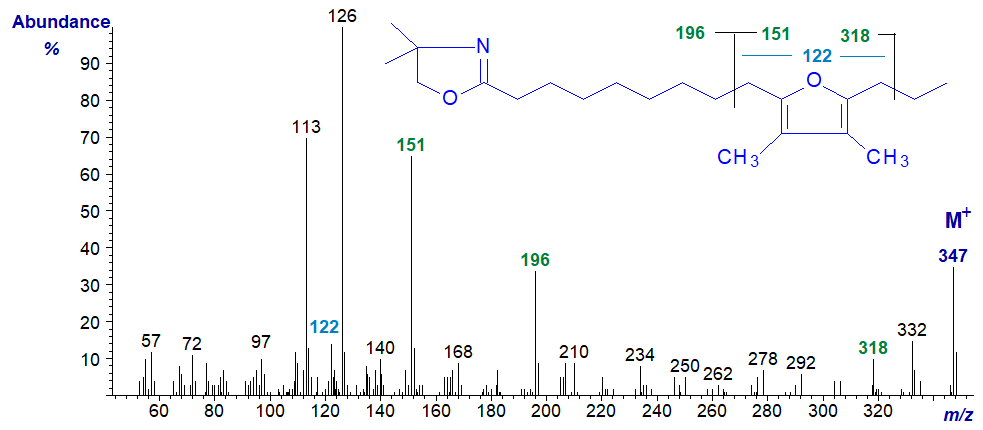
The spectra of the pyrrolidine and DMOX derivatives of 10,13-epoxy-11,12-dimethyl-hexadecadienoate follow. They again show the same diagnostic fragments indicated on the figures although the relative intensities differ appreciably. One difference from the previous two spectra is the ion at m/z = 122 for a ring structure with two methyl groups attached.
Alkoxy Fatty Acids
Methoxy fatty acids are often found in the lipids of marine invertebrates and in some bacteria, but rarely elsewhere. As part of a research programme, a methoxy derivative was synthesised from ricinoleic acid of castor oil, and the mass spectrum of the pyrrolidide of 12‑methoxy-octadec-9-enoate is -
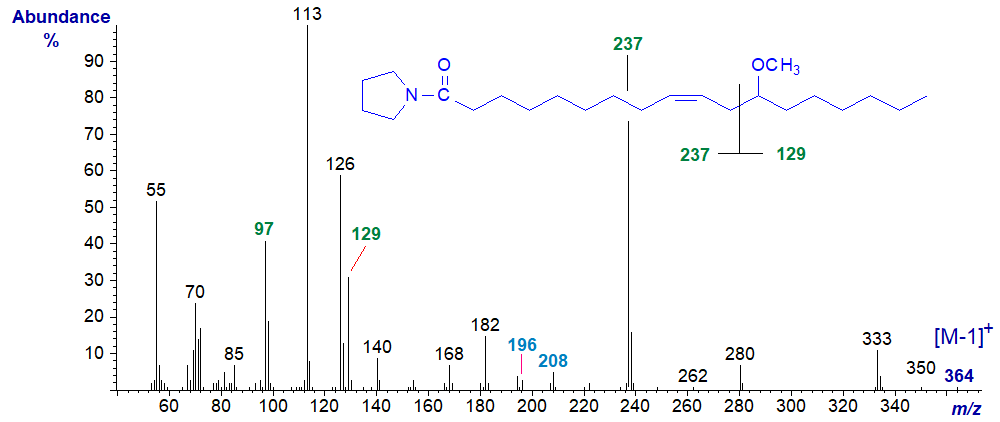
There is a rather simple cleavage between carbons 11 and 12 as indicated to give fragments at m/z = 129 and 237. The latter loses the elements of methanol to yield the ion at m/z = 97 (cf., the spectrum of the methyl ester). The double bond is located by the gap of 12 amu between m/z = 296 and 208 as with other monoenes as the pyrrolidides.
The fatty acids whose spectra are illustrated here next were formed as artefacts while attempting to hydrolyse or methylate brominated fatty acids produced from brominated hydrocarbons by microorganisms (with Dr Jack Hamilton of Queens University, Belfast). None of these spectra have been published elsewhere.
DMOX derivative of 17-ethoxy-heptadec-9-enoate -
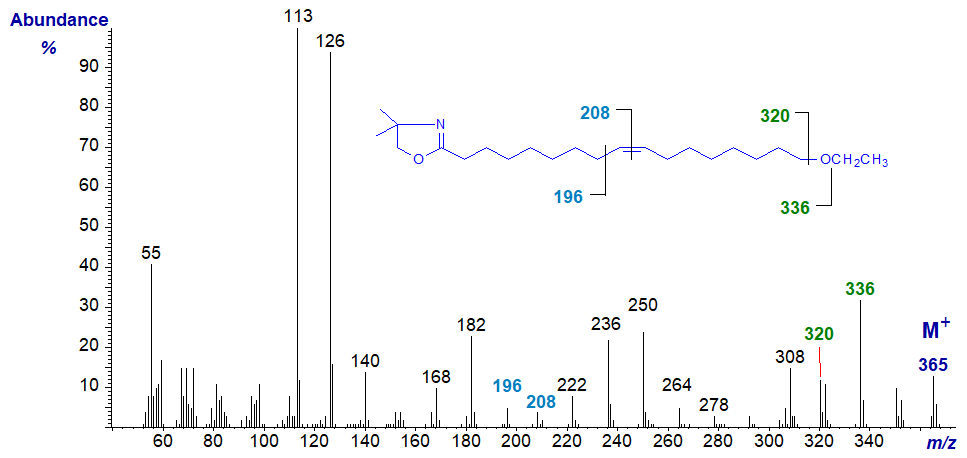
In this instance, the double bond in position 9 is easily located by the gap of 12 amu between m/z = 196 and 208 (see the web page on DMOX derivatives of monoenes), although the fragmentations around the terminal ethoxyl moiety are less clear cut than with 3‑pyridylcarbinol esters because of the competing reaction for loss of a methyl group from the dimethyloxazoline ring (Hamilton and Christie, 2000). Nonetheless, the gaps of 29 amu and 16 amu from the molecular ion for the ethyl and oxygen moieties, respectively, can be discerned as marked on the spectrum.
The mass spectrum of the DMOX derivative of 17-methoxy-octadec-9-enoate is -
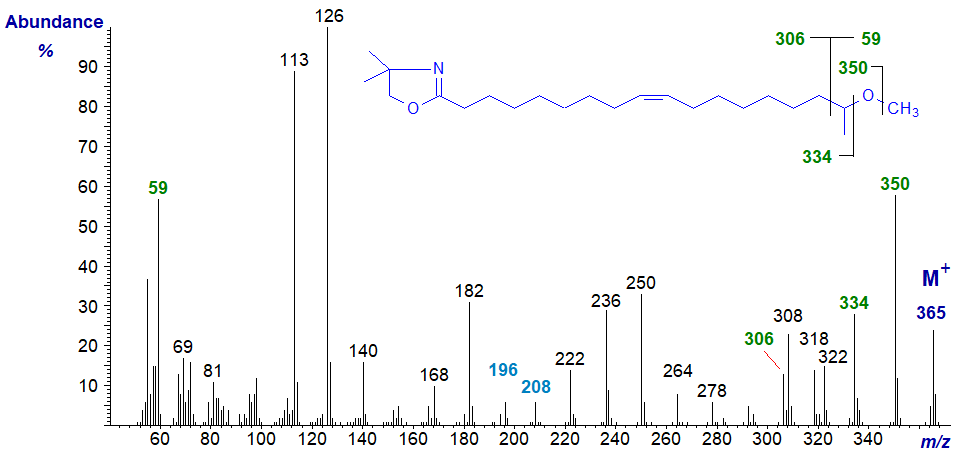
As with the previous spectrum, following the molecular ion, there is a gap of 15 amu for the loss of the methyl group from the methoxyl moiety to m/z = 350, followed by a gap of 16 amu for the oxygen atom. Thereafter, the spectrum is somewhat complicated, presumably because of competing fragmentations in which methyl groups are lost from the oxazoline ring. We would not expect this to be a problem with pyrrolidine derivatives but have no spectra available for confirmation. The double bond is located by the gap of 12 amu between m/z = 196 and 208, as in the previous spectrum. The abundant ion at m/z = 59 from the terminal part of the molecule is present in the spectra of the DMOX derivatives of related fatty acids.
There are more spectra of DMOX derivatives of methoxy and ethoxy fatty acids in our Archive section, but without interpretation.
References
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Imbs, A.B., Demidkova, D.A., Dautova, T.N. and Latyshev, N.A. Fatty acid biomarkers of symbionts and unusual inhibition of tetracosapolyenoic acid biosynthesis in corals (Octocorallia). Lipids, 44, 325-335 (2009); DOI.
- Marx, F. and Classen, E. Analysis of epoxy fatty acids by GC-MS of their dimethyloxazoline derivatives. Fett/Lipid, 96, 207-211 (1994).
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
