GC-MS of Fatty Acid Derivatives
Choice of Columns
1. General Considerations
 The choice
of a column for gas chromatography (GC) of fatty acid derivatives and other lipids is a difficult one for newcomers to the topic.
The literature supplied by manufacturers can be confusing if not actually in error at times, and certainly, the advice given is never objective.
It is impossible for me to describe or review the various types of column available from individual manufacturers,
as we have only been able to try out a relative few in our work, and I have been out of touch for many years.
If I mention a particular column from one manufacturer, it should not be taken as an unqualified endorsement of that product
- simply because I have not tried all the competition.
Please consider the general principles rather than the specifics.
Companies are constantly refining and improving their manufacturing processes, sometimes changing phase properties a little.
Readers can find further information in my book [1].
The choice
of a column for gas chromatography (GC) of fatty acid derivatives and other lipids is a difficult one for newcomers to the topic.
The literature supplied by manufacturers can be confusing if not actually in error at times, and certainly, the advice given is never objective.
It is impossible for me to describe or review the various types of column available from individual manufacturers,
as we have only been able to try out a relative few in our work, and I have been out of touch for many years.
If I mention a particular column from one manufacturer, it should not be taken as an unqualified endorsement of that product
- simply because I have not tried all the competition.
Please consider the general principles rather than the specifics.
Companies are constantly refining and improving their manufacturing processes, sometimes changing phase properties a little.
Readers can find further information in my book [1].
Narrow-bore fused-silica columns are robust, and with care they will last for one to two years, while affording high resolution and low adsorptivity with quantitative recovery. Although it may be tempting for anyone new to the technique to purchase columns of 50 metres in length to obtain the maximum resolution possible, excellent results can be obtained with columns of 10 or 25 metre with most samples. One advantage of shorter columns indeed is that analysis times can be reduced appreciably, so that more samples can be analysed in a given time. For analytical purposes, the internal diameter of the column should be 0.2 to 0.3 mm, though columns of 0.1 mm permit rather short analysis times (3 to 7 min).
2. Routine Analysis of Methyl Esters of Fatty Acids
The liquid phases in use for the GC analysis of methyl ester derivatives of fatty acids are almost exclusively polar polyesters, although a few applications for thermally stable non-polar silicone phases remain. With non-polar phases, unsaturated components are eluted before the saturated derivatives with the same chain-length, while with polar phases the reverse is true, i.e., unsaturated components are eluted after saturated. Polyesters can be classified according to their degree of polarity, and in current practice only two main types need be considered, i.e., those of low to medium polarity like the Carbowax type (polyethylene glycol under various trade names) and those of high polarity, such as CPSil-88TM, BPX70TM, SP-2340TM or SLB-IL111TM.
Changing the polarity of a polyester phase does not change the order of elution of components within a given chain-length group, but it can affect the elution order relative to components of other chain lengths as indicated in Figure 1. As mentioned briefly above, unsaturated fatty acid derivatives elute before the corresponding saturated on non-polar silicone phases, and resolution may not be adequate, e.g., 18:2(n-6) and 18:3(n‑3) may elute together, and indeed in some instances, the normal order can be reversed.
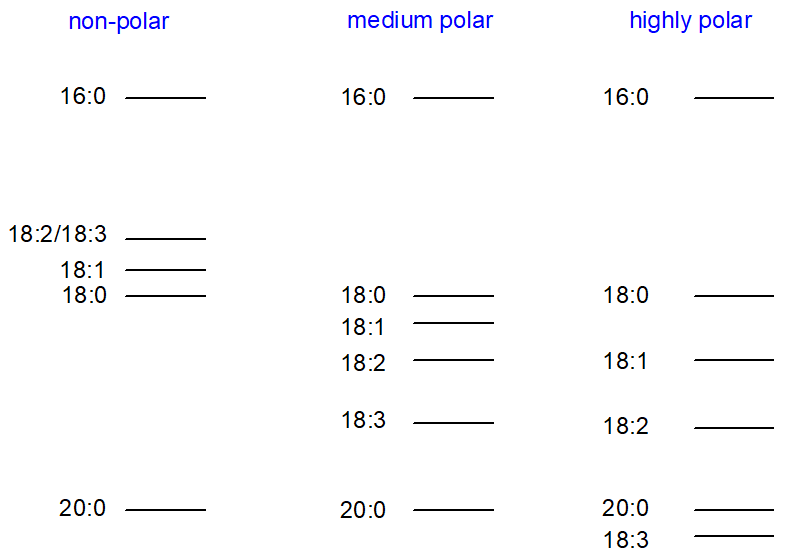
Figure 1. Order of elution of fatty acid methyl esters on GC columns of varying polarities.
It is possible to eliminate the difficulties with overlapping components of different chain-lengths by using polyester liquid phases of low to medium polarity. The author has favoured phases of the Carbowax type for the analysis of fatty acids of animal origin, as this gives satisfactory resolution of all the important polyunsaturated fatty acids with no interference by components differing in chain-length by two carbon atoms, at least in the important C18 and C20 regions. For most practical purposes, positional isomers of unsaturated fatty acids are well resolved. Ackman [2] has proposed that phases of the Carbowax 20MTM type "should be utilized in the 'standard' reference WCOT column for inter-laboratory studies as well as for application in its own right". For the moment and until any new phase with demonstrable advantages is introduced, this suggestion seems eminently sensible. My former colleagues use a 25-metre column (0.25 mm i.d.) of this kind for all routine analysis of clinical, seed oil and fish oil samples. This should be the first type of column anyone should buy for analysis of the common range of fatty acids of most origins.
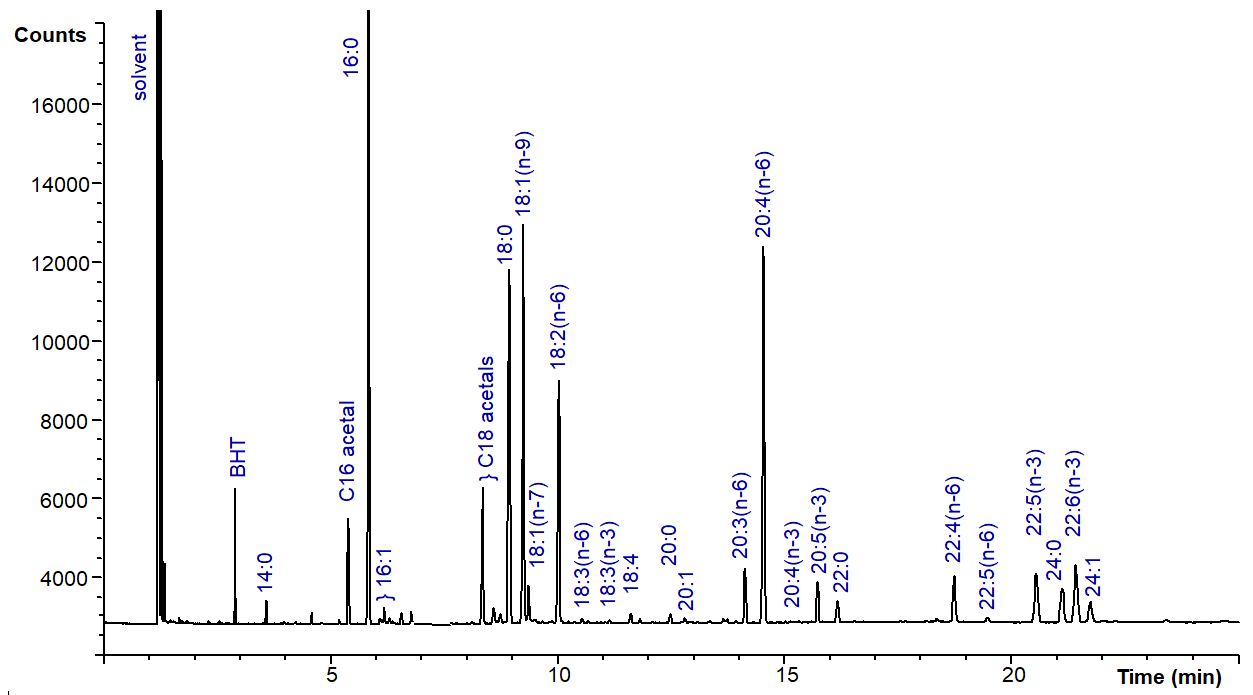
Lipid analysts soon acquire an intuitive understanding of the relationship between the retention times of peaks on a GC trace and their identity. A typical fingerprint of the fatty acids from animal tissue phospholipids on a column of the Carbowax type would have the 16:0 component standing in relative isolation, followed by the three peaks for the C18 components (18:0, 18:1 and 18:2), then a gap to the next substantial peak for 20:4(n-6), followed by a further gap to the C22 components, the last of which is 22:6(n-3). Some care in the selection of operating conditions may be necessary to ensure separation of 22:5/22:6 from 24:0/24:1. Many of the minor peaks can be identified tentatively according to their proximity to these major components. Figure 2 illustrates a typical chromatogram of the fatty acids of human erythrocytes as methyl esters on a column of the Carbowax type (25 m) with flame-ionization detection.
Figure 2. Separation of the fatty acids of human erythrocytes as the methyl esters by GC on a column of the Carbowax type. The column (25 m x 0.25 mm x 0.2 μm) was coated with CP-Wax 52CBTM (Agilent Inc.); the oven temperature was held at 170°C for 3 min, then was raised by 4°C/min to 220°C. Hydrogen was the carrier gas at a flow rate of 1 mL/min.
Excellent separations are achieved of fatty acid esters of a given chain-length that differ both by degree of unsaturation and in the positions of the double bonds, and two isomers of 18:1 and of 18:3 are separated, as are three isomers of 20:3, two of 20:4 and two of 22:5. With the methyl esters of the more common families of polyunsaturated fatty acids, the shorter the distance between the last double bond and the end of the molecule, the longer the retention time of the isomer.
Even better separations of positional isomers are obtainable with a more polar phase, though the order of elution relative to components of a different chain length may vary, and there can be other problems of components overlapping. Readers might check a publication that illustrates what can be achieved when the best possible separations are required (SLB-IL111TM phase; 200 m x 0.25 mm) [3]; in combination with silver ion HPLC, 125 components were identified in menhaden oil. Manufacturers tend to give prominence to these high polarity phases in their literature and recommend them for routine fatty acid analysis. I disagree. Apart from cost and the fact that such resolution is rarely necessary, their main disadvantage is that there is some overlap in the range of elution of fatty acids of different chain lengths, and the nature and extent of the problem can be rather sensitive to column temperature or the temperature gradient [4]. For example, it is possible to change the order of elution of 20:1 and 18:3(n-3) methyl esters on the columns of highest polarity by modifying the column temperature. This does not appear to be a significant problem with columns of the CarbowaxTM type. On the other hand, with mass spectrometric detection and identification, the order of elution is much less relevant.
3. Gas Chromatography with Mass Spectrometry
As a rule, there are few problems in using most of the commercial columns and stationary phases already in wide-spread use for the analysis of methyl ester derivatives of fatty acids in the range C14 to C22 by mass spectrometry. Problems can arise with samples that are a little out of the ordinary, such as those containing fatty acids of very-long-chain-length, and especially when 3-pyridylcarbinol (picolinyl) esters, pyrrolidides and 4,4‑dimethyloxazoline (DMOX) derivatives must be analysed by GC-MS. Bleeding of the stationary phase at higher temperatures can then lead to troublesome background noise in mass spectra.
Several manufacturers now make columns with cross-linked highly stable phases of from low to high polarity specifically for use with mass spectrometry. As the standard column in our laboratory, we have made extensive use of Supelcowax 10™ (25 m in length; from Supelco-Sigma Inc.). It is typical of columns of the Carbowax type in its elution properties and can cover with ease a wide range of fatty acid methyl esters or DMOX derivatives (up to C30 in chain-length). Comparable phases are no doubt available from other suppliers. 3-Pyridylcarbinol esters and pyrrolidides are more polar or have higher molecular weights, and the Supelcowax column can cope with the common range up to about C22.
 When
a more polar phase is required, as for separation of cis/trans isomers, we have used a 100m column of CPSil-88TM
(from Agilent Inc.), which presents no problems of background noise for methyl esters of C14 to C22 fatty acids.
We have also used a column with the polar phase BPX-70TM (from SGE Ltd) for 3-pyridylcarbinol esters and pyrrolidides.
Again, other manufacturers have columns available that fully meet the requirements for GC-MS,
but we have no practical experience of any other than those listed.
When
a more polar phase is required, as for separation of cis/trans isomers, we have used a 100m column of CPSil-88TM
(from Agilent Inc.), which presents no problems of background noise for methyl esters of C14 to C22 fatty acids.
We have also used a column with the polar phase BPX-70TM (from SGE Ltd) for 3-pyridylcarbinol esters and pyrrolidides.
Again, other manufacturers have columns available that fully meet the requirements for GC-MS,
but we have no practical experience of any other than those listed.
Finally, we need a column coated with a low polarity silicone phase because of its high thermal stability. Such columns lack the resolution of phases of higher polarity but are adequate for many purposes. Thus, we use a DB5TM column (25 m, Agilent Inc.) for fatty acids of longer than usual chain-length when they are in the form of the 3‑pyridylcarbinol or pyrrolidide derivatives, for hydroxy fatty acids, and for other lipids of relatively high molecular weight, including sterols and waxes. It is essential for any fatty acid derivatives or other lipids that have been converted to the trimethylsilyl ether or related derivatives, as excess derivatization agent can degrade polyester phases.
It is not always possible to expect the same quality of resolution in mass spectrometry applications as with a flame-ionization detector (FID), mainly because of the greater dead volume in a mass spectrometer in comparison with an FID. This depends largely on the specific instrument in use, so further comment is impossible here. With 3‑pyridylcarbinol esters and pyrrolidides, you should not expect the same resolution as with methyl esters and DMOX derivatives, because of the high molecular weight and polarity of the former. However, pyrrolidides do have some interesting and unusual GC properties (see the appropriate web page).
4. Practical Considerations
As a practical point, the working lives of columns can be prolonged if they are used continuously and not removed from the instrument, and this seems to be especially true of the highly polar phases when in 100 metre lengths. The latter are often made as two 50 metre columns coupled together, and the coupling can be rather fragile in our experience; it is sensitive to physical handling (installing and removing from a GC oven) and to temperature extremes. In view of the high costs of such columns, analysts should be aware of this potential problem. When a column is not in use, a flow of inert carrier gas should be maintained with the column held at a temperature of 40-50°C.
References
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Ackman, R.G. WCOT (capillary) gas-liquid chromatography. In: Analysis of Oils and Fats. pp. 137-206 (1986) (edited by R.J. Hamilton & J.B. Rossell, Elsevier Applied Science, London).
- Fardin-Kia, A.R., Delmonte, P., Kramer, J.K.G., Jahreis, G., Kuhnt, K., Santercole, V. and Rader, J.I. Separation of the fatty acids in menhaden oil as methyl esters with a highly polar ionic liquid gas chromatographic column and identification by time of flight mass spectrometry. Lipids, 48, 1279-1295 (2013); DOI.
- Wolff, R.L. Analysis of α-linolenic acid geometrical isomers in deodorized oils by capillary GC on cyanoalkyl polysiloxane stationary phases: a note of caution. J. Am. Oil Chem. Soc., 71, 907-909 (1994); DOI.
| © Author: William W. Christie |  |
|
| Updated: January 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.