Concentration of Minor Fatty Acid Components
before Analysis by GC-MS
Determination of structures of fatty acids by GC-MS is not always straightforward. Derivatized fatty acids may not be sufficiently well resolved by gas chromatography for analysis by mass spectrometry, and minor components may be obscured, so analysis of the latter is greatly assisted if they can be concentrated by some means. Urea fractionation is a useful technique for concentration of polyunsaturated, branched-chain or cyclic fatty acids in relatively large amounts. However, chromatographic techniques, such as high-performance liquid chromatography (HPLC) in the reversed-phase mode or silver ion chromatography (either high-performance, column or thin-layer chromatography (TLC) afford much greater versatility, selectivity and resolution, while counter-current distribution equipment is now being used successfully for the purpose. The choice of the most appropriate method will depend on the nature of the sample, the amount of material available and the equipment available to the analyst. Several published review articles and books on the topic are available [1-3].
1. Urea Fractionation
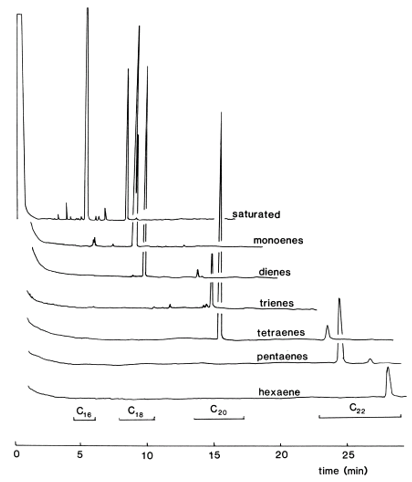
When urea is permitted to crystallize in the presence of fatty acid methyl esters, it forms hexagonal crystals with a channel into which some fatty acids may be able to fit and so be removed from solution. Saturated straight-chain acids (as the methyl ester derivatives) form such urea inclusion complexes readily, but the double bonds of unsaturated fatty acids or the presence of methyl branches increase their bulk so that they form complexes less readily or not at all. Monoenoic fatty acids are complexed to a limited extent, but polyenes are not. When the crystallization process is complete, the saturated and a proportion of the monoenoic fatty acids are locked up in the crystals, while polyunsaturated, branched-chain, and cyclic fatty acids remain in the mother liquor. Figure 1 illustrates a GC separation of the polyunsaturated fraction (non-adducted) of methyl esters from a fish oil; traces only of saturated or monoenoic components remain. Although the procedure is sometimes carried out on large amounts of material, even on an industrial scale, the following simplified method is of more value to analysts [4]. With care, there is relatively little opportunity for harm to come to polyunsaturated esters.
| Laboratory protocol: The methyl esters (up to 100 mg) are dissolved in hexane (4 mL), and urea (1.5 g) moistened with methanol (15 drops) is added. After standing overnight, the solid is filtered off and thoroughly washed with hexane; these washings and the hexane filtrates are combined and washed with water to remove any residual urea. The hexane layer is dried over anhydrous sodium sulfate and evaporated, yielding a branched-chain and/or polyunsaturated fraction. |
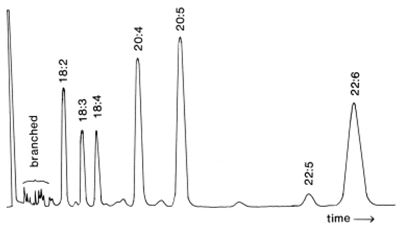 |
Figure 1. GC trace of polyunsaturated fraction (non-adducted) from methyl esters of a fish oil. |
2. Solvent Partition in the Form of Silver Ion Complexes
Although silver ion complexation is usually used in conjunction with chromatography to separate unsaturated compounds (see below), a simple solvent partition procedure has described that permits the isolation of a concentrate of polyunsaturated fatty acids [5].
| Laboratory protocol: The fatty acid methyl esters (up to 1 g) in 2,2,4-trimethylpentane (10 mL) are partitioned with vigorous shaking with the same volume of ethanol-water (1:1) containing silver nitrate (2.5 g). The upper organic layer, which contains mainly saturated and monoenoic components, is removed. The lower layer is diluted with water (10 mL) and is extracted with hexane (3 x 10 mL). The hexane extract is dried over anhydrous sodium sulfate and evaporated to yield the polyunsaturated esters. |
Note: Take great care to prevent and contain all spillages, which can leave an indelible stain on laboratory benches and equipment. Wear protective gloves to prevent the silver ion solution from touching the skin.
3. Solid-Phase Extraction Methods
Fatty acid methyl esters from fish oils (5 mg scale) have been divided into two fractions, i.e., saturated plus monoenes and polyunsaturated, on a solid-phase extraction column with bonded aminopropyl groups [6], and similar methodology is used for hydroxy/non-hydroxy acids [7], while solid-phase extraction columns of the reversed-phase type have been employed for enrichment of methyl eicosapentaenoate from algal fatty acids [8]. We have no experience of these methods so can offer no further guidance.
Bond Elut SCX™ SPE columns (Agilent) packed with a silica-based benzenesulfonic acid medium can be converted to the silver ion form and used to achieve useful separations of methyl ester derivatives of fatty acids on a small scale (~250 μg), which can be sufficient for analysis by GC-MS [9]. No silver salts are eluted with the fractions from columns of this type.
Laboratory protocol: A solution of silver nitrate (20 mg) in acetonitrile-water (0.25 mL; 10:1, v/v)
is allowed to flow through a Bond Elut SCX™ cartridge (0.5 g adsorbent), wrapped to the level of the top of the sorbent bed
in aluminium foil to exclude light; the SPE column is flushed with acetonitrile (5 mL), acetone (5 mL)
and dichloromethane (10 mL) and is then ready for use.
These solvents in various proportions are employed in the optimum elution scheme for the isolation of fractions as listed in the table below.
A methyl ester sample (0.1 to 0.5 mg) is applied to the column in a small volume of dichloromethane.
Solvent mixtures are allowed to flow under gravity.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
As substantial changes in solvent composition are utilized at each step, there is only a little cross-contamination, and this is only significant with the later fractions; satisfactory resolution of components with zero to six double bonds is obtained as illustrated in Figure 2. It is easy to see that the simplified fractions thus obtained are much easier to analyse by GC/MS, especially for minor components. It is important that the columns should not be overloaded or that the flow-rate be increased artificially otherwise resolution is lost. Some modification to these conditions may be required for different commercial brands of column, or with the pre-silvered columns, which can now be purchased from Supelco/SigmaTM (Discovery Ag+-Ion SPE columnsTM). The latter are now marketed for the separation and analysis of trans isomers, and several interesting applications have been published in which somewhat different elution conditions from those above were employed (cf., [10,11], for example).
4. Silver Ion TLC and HPLC
Silver ion HPLC and TLC are invaluable techniques for separating fatty acids according to the number and configuration of the double bonds, but as my former collaborator Boryana Nikolova-Damyanova and I produced a series of web pages on this technique for the AOCS Lipid Library, some brief details only are sketched out here. It is easy to see that the simple fractions thus obtained are much easier to analyse by GC linked to mass spectrometry. Substantial reviews by Nikolova-Damyanova and others have been published on the topic [12-14].
In short, silver ion TLC is simple to undertake using silica gel layers into which silver nitrate is incorporated immediately before use. Silver ion HPLC uses columns containing ion-exchange materials with bonded phenylsulfonic acid groups to which silver can be linked. It is not difficult to prepare suitable columns in the laboratory [15] (although I have heard doubts on the reliability of some manufacturers' phases of the required type), or they can be purchased ready made from Agilent (ChromSpher Lipids™ columns). Adaptations of silver ion methodology to simple partition and solid-phase extraction techniques are described above.
5. Reversed-Phase HPLC
Although HPLC in the reversed-phase mode is sometimes used for quantitative analysis of fatty acids, it is of much more value when connected to mass spectrometry (although I have no practical experience to recount), and it can be recommended for oxylipins or other fatty acids that contain labile functional groups. It is also useful for micro-preparative purposes, i.e., to isolate components for further analysis by GC-MS. The technique has been reviewed by Nikolova-Damyanova [16].
Octadecylsilyl (ODS)-bonded phases have become the standard for the purpose, and those that have been stabilized against attack by bases are useful for separation both of methyl esters and of the nitrogen-containing derivatives used in mass spectrometry. In our work, we used a column of Hichrom RPB™, but comparable types from other suppliers should be suitable. Conventional ODS columns can be used with nitrogen-containing derivatives provided that small amounts of volatile amines are incorporated into the mobile phase [17].
In reversed-phase HPLC, unsaturated fatty acids are eluted substantially ahead of the saturated fatty acid with the same chain-length, each double bond reducing the retention time by the equivalent of approximately two carbon atoms. Thus, oleic acid derivatives tend to elute in the same region as the corresponding palmitic acid derivatives, though the two are usually well resolved. Indeed, the resolving power of columns packed with modern micro-particulate phases is such that there need be little overlap of the main components of interest in natural samples if suitable mobile phases are selected. On the other hand, because of the nature of the separation, different fatty acids are easily confused, and it is necessary to exercise care to ensure correct identification. Model mixtures of pure standards are invaluable in developing separations and as an aid to the identification of unknowns.
A separation of 3-pyridylcarbinol ('picolinyl') esters prepared from the fatty acids of borage oil, on a column of Hichrom RPB™ using acetonitrile only as the mobile phase is illustrated [2]. In this example, the more highly unsaturated C18 esters elute first, followed by 16:0, then the C20 monoenoic fatty acids and 18:0.
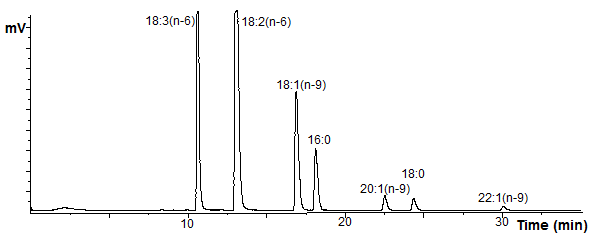
Figure 3. Reversed-phase HPLC separation of 3-pyridylcarbinol esters. |
| Laboratory protocol: To reproduce this separation, a column of Hichrom RPB™ (250 x 4.6 mm; Hichrom Ltd, Reading, UK) or equivalent is used with acetonitrile as mobile phase, with the flow rate programmed from 0.5 to 1.5 mL/min over 30 min and held at this for a further 5 min. The temperature of the column is maintained at 20°C. The sample of 3-pyridylcarbinol esters is injected in a solution of acetone-acetonitrile (5 μL, 1:9, v/v). UV detection at 254 nm, or an evaporative light-scattering detector with stream-splitter can be used. |
If the column temperature is kept constant, the elution times are highly reproducible, but this is not essential to the separation. Here, evaporative light-scattering detection was used for convenience, but UV detection should be possible by using the specific absorbance of the pyridine ring. Methyl esters and DMOX derivatives can be separated under the same conditions, except that corresponding components elute a little earlier than for 3‑pyridylcarbinol esters, presumably because the last are a little more hydrophobic. Again, UV detection should be possible for methyl esters with the above elution system, but at 206 nm. It should be noted that base-deactivated columns of this type are rendered unusable by exposure to acids.
6. Counter-Current Distribution
Simple bench-top counter-current distribution equipment is now available that permits separation of fatty acid derivatives under mild conditions on a much larger scale than is possible with conventional HPLC equipment. Separations are based upon both chain-length and degree of unsaturation. Although the resolution is probably not as good as reversed phase HPLC, minor components may be obtained more easily when sample size is not a limiting factor. We have no experience of the technique so cannot comment further, but publications from Professor Walter Vetter’s laboratory show what can be achieved [18,19]. He has reviewed the technique on-line in the AOCS Lipid Library here...
References
 Christie, W.W.
Structural analysis of fatty acids. In: Advances in Lipid Methodology - Four, pp. 119-169 (1997)
(edited by W.W. Christie, Oily Press, Dundee).
Christie, W.W.
Structural analysis of fatty acids. In: Advances in Lipid Methodology - Four, pp. 119-169 (1997)
(edited by W.W. Christie, Oily Press, Dundee).- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Linstead, R.P. and Whalley, M. The formation of crystalline complexes between urea and esters, and their application to the separation of mixtures of esters. J. Chem. Soc., 2987-2989 (1950).
- Peers, K.E. and Coxon, D.T. Simple enrichment procedure for the estimation of minor polyunsaturated fatty acids in food fats. J. Food Sci. Technol., 21, 463-469 (1986).
- Wilson, R., Henderson, R.J., Burkow, I.C. and Sargent, J.R. The enrichment of n-3 polyunsaturated fatty acids using aminopropyl solid phase extraction columns. Lipids, 28, 51-54 (1993); DOI.
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere, 35, 275-294 (1997); DOI.
- Cohen, Z. and Cohen, S. Preparation of eicosapentaenoic acid (EPA) concentrate from Porphyridium cruentum. J. Am. Oil Chem. Soc., 68, 16-19 (1991); DOI.
- Christie, W.W. Silver ion chromatography using solid-phase extraction columns packed with a bonded-sulfonic acid phase. J. Lipid Res., 30, 1471-1473 (1989); DOI.
- Santercole, V., Delmonte, P. and Kramer, J.K.G. Comparison of separations of fatty acids from fish products using a 30-m Supelcowax-10 and a 100-m SP-2560 column. Lipids, 47, 329-344 (2012); DOI.
- Belaunzaran, X., Bravo-Lamas, L., Kramer, J.K.G., Morales, R. and Aldai, N. Silver ion solid-phase extraction cartridges employing glass housings overcome the limitations observed in the GC analysis of animal lipids with low trans fatty acid content. Eur. J. Lipid Sci. Technol., 4, 1600124 (2017); DOI.
- Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology - One, pp. 181-237 (ed. W.W. Christie, Oily Press, Dundee) (1992).
- Nikolova-Damyanova, B. Lipid analysis by silver ion chromatography. In: Advances in Lipid Methodology - Five, pp. 43-123 (ed. R.O. Adlof, Oily Press, Bridgwater) (2003).
- Tryon-Tasson, N., Ryoo, D., Eor, P. and Anderson, J.L. Silver-mediated separations: A comprehensive review on advancements of argentation chromatography, facilitated transport membranes, and solid-phase extraction techniques and their applications. J. Chromatogr. A, 1705, 464133 (2023); DOI.
- Christie, W.W. A stable silver-loaded column for the separation of lipids by high-performance liquid chromatography. J. High Resolut. Chromatogr., Chromatogr. Commun., 10, 148-150 (1987); DOI.
- Nikolova-Damyanova, B. Reversed-phase HPLC: general principles and application to the analysis of fatty acids and triacylglycerols. In: Advances in Lipid Methodology - Four, pp. 193-251 (ed. W.W. Christie, Oily Press, Dundee) (1997).
- Christie, W.W. and Stefanov, K. Separation of picolinyl ester derivatives of fatty acids by high-performance liquid chromatography for identification by mass spectrometry. J. Chromatogr. A, 392, 259-265 (1987); DOI.
- Hammann, S., Tillmann, U., Schröder, M. and Vetter, W. Profiling the fatty acids from a strain of the microalgae Alexandrium tamarense by means of high-speed counter-current chromatography and gas chromatography coupled with mass spectrometry. J. Chromatogr. A, 1312, 93-103 (2013); DOI.
- Schröder, M. and Vetter, W. Detection of 430 fatty acid methyl esters from a transesterified butter sample. J. Am. Oil Chem. Soc., 90, 771-790 (2013); DOI.
| © Author: William W. Christie |  |
|
| Updated: March 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.