Mass Spectrometry of Methyl Esters
Allenic Fatty Acids
 Fatty acids
with allenic fatty acid systems are rather uncommon in nature, and we have not encountered them as such in our work, but
we do have spectra from a series of synthetic allenes, both as the 3-pyridylcarbinol ('picolinyl') and methyl esters.
As these spectra are unique and have relevance to the interpretation of the spectra of acetylenic fatty acids,
selected examples are worth illustrating with a minimal commentary.
Further details are available in the published paper, but more spectra can be illustrated here than is possible in a printed publication
(Christie, W.W., Brechany, E.Y., Lie Ken Jie, M.S.F. and Wong, C.F. Mass spectrometry of derivatives of isomeric allenic
fatty acids. Biol. Mass Spectrom., 21, 267-270 (1992);
DOI).
Here the spectra of methyl esters are discussed, while those of
3-pyridylcarbinol esters are described in a separate document.
More general mechanistic aspects are discussed on or web page dealing with methyl esters of saturated
fatty acids.
There is an account of the natural occurrence of allenic fatty acids in the
Lipid Essentials section of this website.
Fatty acids
with allenic fatty acid systems are rather uncommon in nature, and we have not encountered them as such in our work, but
we do have spectra from a series of synthetic allenes, both as the 3-pyridylcarbinol ('picolinyl') and methyl esters.
As these spectra are unique and have relevance to the interpretation of the spectra of acetylenic fatty acids,
selected examples are worth illustrating with a minimal commentary.
Further details are available in the published paper, but more spectra can be illustrated here than is possible in a printed publication
(Christie, W.W., Brechany, E.Y., Lie Ken Jie, M.S.F. and Wong, C.F. Mass spectrometry of derivatives of isomeric allenic
fatty acids. Biol. Mass Spectrom., 21, 267-270 (1992);
DOI).
Here the spectra of methyl esters are discussed, while those of
3-pyridylcarbinol esters are described in a separate document.
More general mechanistic aspects are discussed on or web page dealing with methyl esters of saturated
fatty acids.
There is an account of the natural occurrence of allenic fatty acids in the
Lipid Essentials section of this website.
Allenic systems are more stable and less prone to migrate than conventional double bonds under electron-impact ionization, so that mass spectra of methyl esters of allenic acids are quite informative. In general, the molecular ion tends to be small but distinct, and there is usually an ion at [M−31]+ for the loss of a methoxyl group. Three useful ions are formed by mechanisms that can be considered simplistically as cleavage on the far side of the molecule beta to the double bond system, at least for the 3,4- to 10,11-isomers, although the relative intensities of the relevant ions vary with the distance from either end of the molecule. They include all or part of the carboxyl group, as shown in the figure (ions 'a, b and c'). When the allenic moiety is further down the chain, a fragment from the distal portion become more significant (ion 'd').
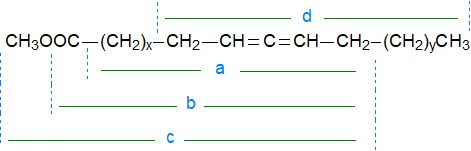 |
| Figure 1. Fragmentation patterns for allenic methyl esters. |
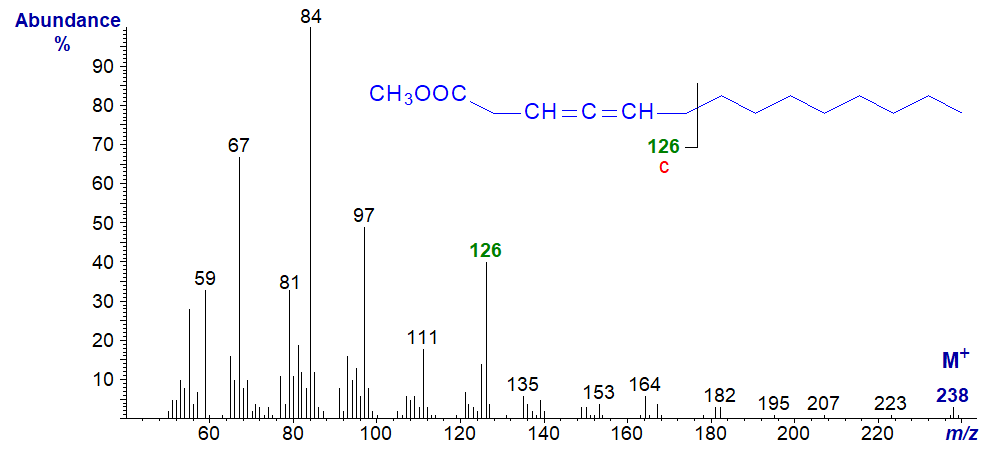
In the spectrum of methyl 3,4-tetradecadienoate, ions 'a', 'b' and 'c' are at m/z = 66, 94 and 126, respectively, although only the last stands out from the background. The ion at m/z = 164 or [M−74]+ represents the loss of the McLafferty ion (cf., the spectrum of the ethyl ester).
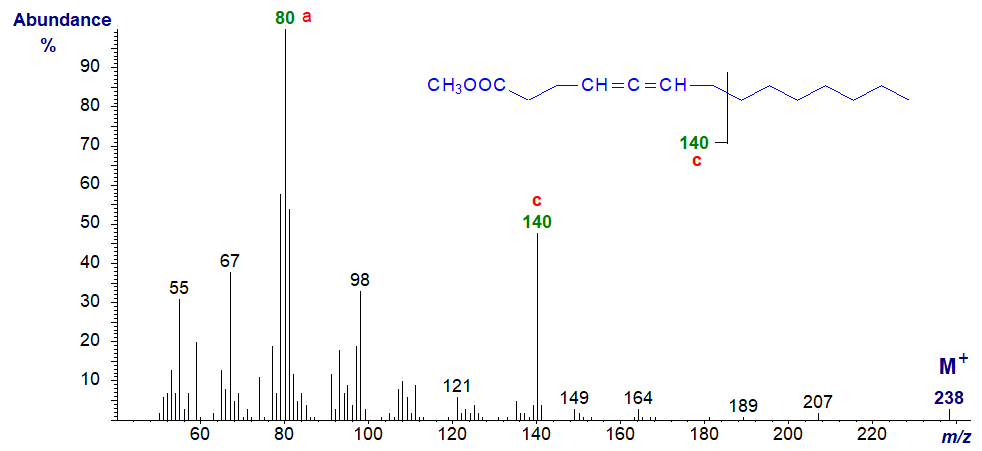
Ions 'a' and 'c' are both abundant in the spectrum of methyl 4,5-tetradecadienoate -
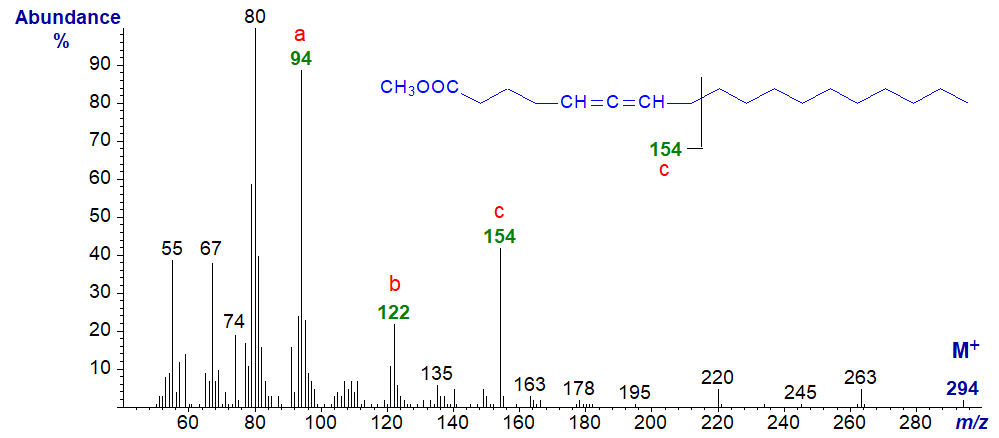
However, with the spectrum of methyl 5,6-octadecadienoate and that of the next isomer, all three ions are distinctive. This spectrum is remarkable similar to that of methyl octadec-6-ynoate (or see our web page on mass spectra of acetylenic acids).
Mass spectrum of methyl 7,8-octadecadienoate -
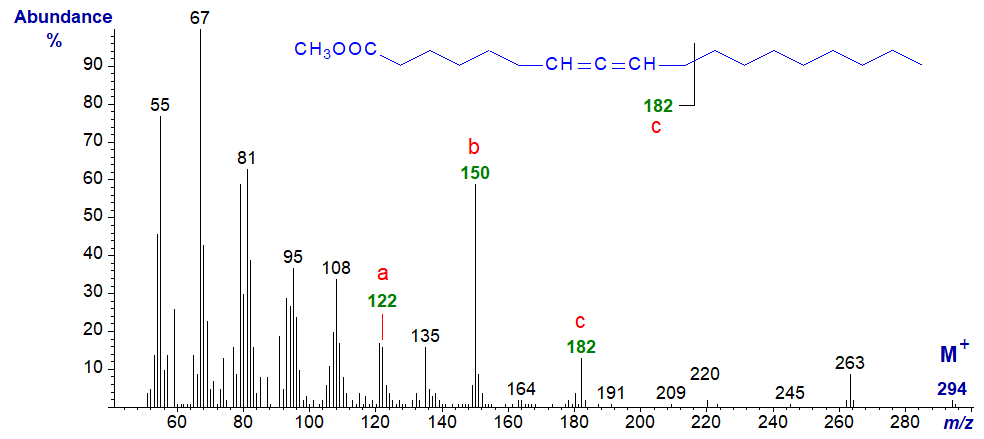
In the mass spectrum of methyl 9,10-octadecadienoate, an ion that probably represents a fragment from the terminal part of the molecule (at m/z = 152) now becomes a useful diagnostic aid, while ions 'a, b and c' are now less abundant. This spectrum and that of the 8,9-isomer have only a superficial resemblance to that of methyl octadec-9-ynoate.
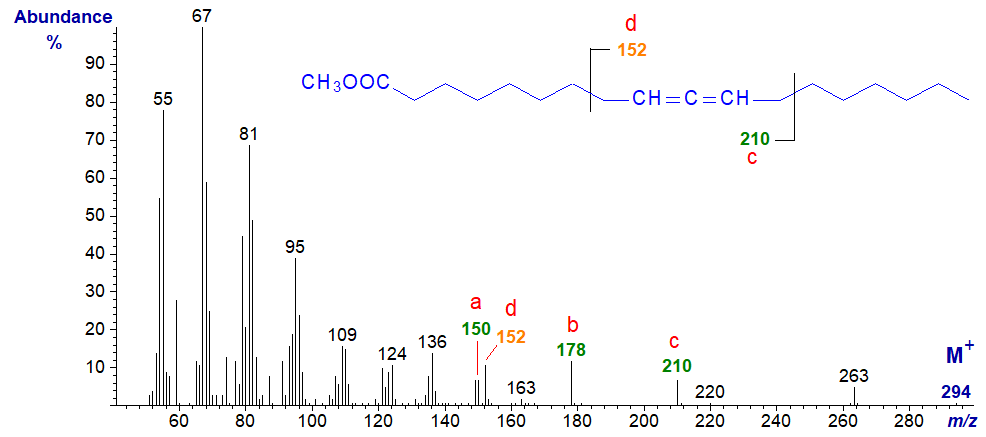
Mass spectrum of methyl 11,12-octadecadienoate -
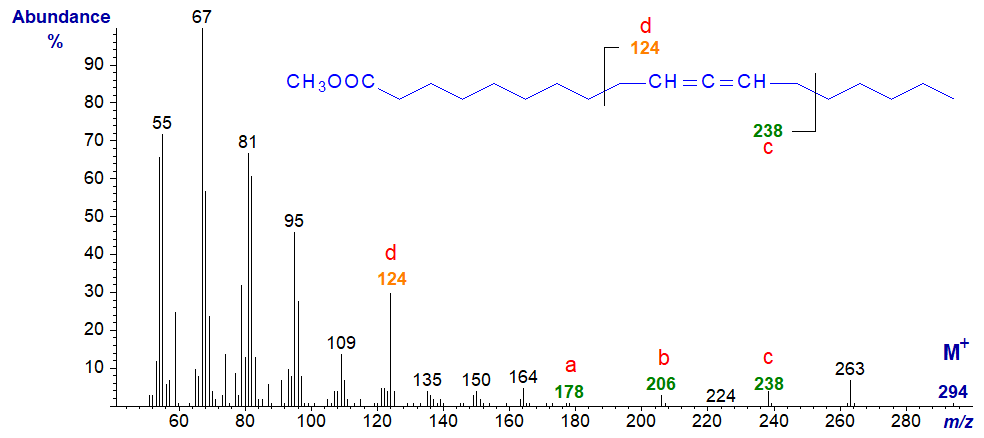
Mass spectrum of methyl 13,14-octadecadienoate - now only the ion 'd' at m/z = 96 from the distal portion of the molecule is significant.
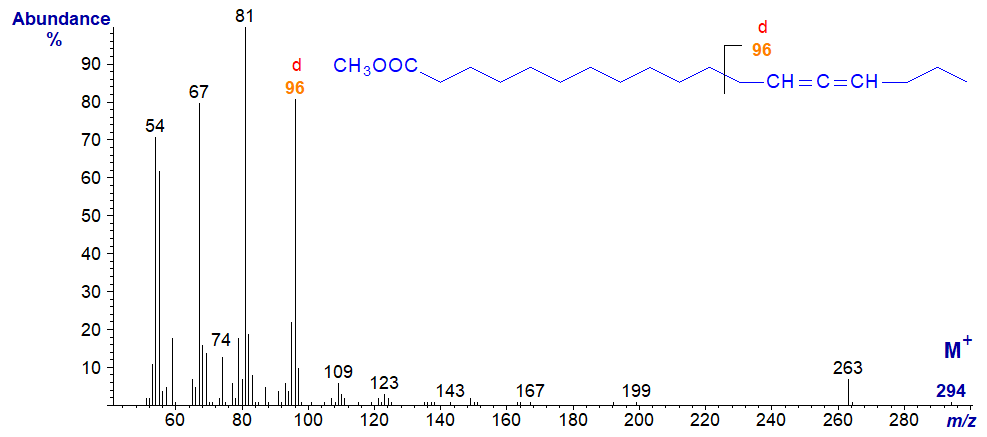
The same feature is seen even when the allene group is in the terminal position as with the spectrum of methyl 12,13-tetradecadienoate -
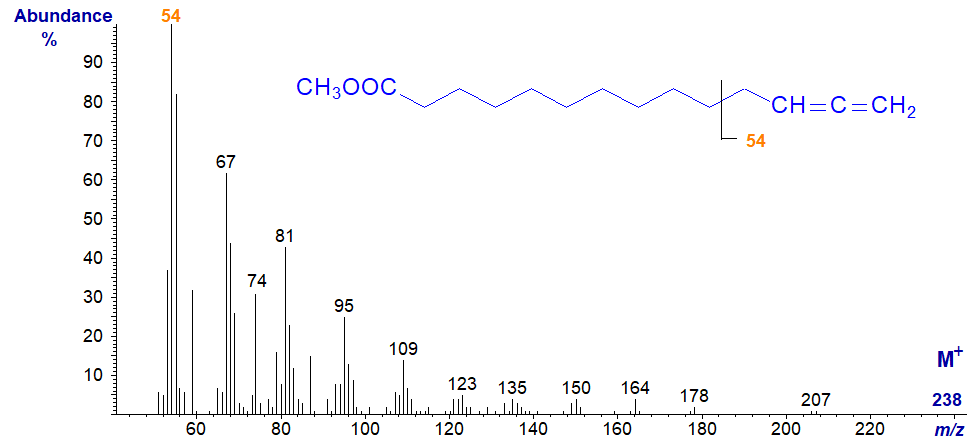
I have not attempted to speculate on the origins of many of the ions in these spectra, as once these are in print, they tend to be given undue credence by readers. This is intended as a practical as opposed to a mechanistic guide, but I hope readers will try to interpret the spectra further.
We have mass spectra on file for methyl esters of further allenic fatty acids, and these can be found in our Archive page, but without interpretation. In addition, the spectra of ethyl 2,3-tetradecadienoate (no other derivative is available for this isomer), ethyl 3,4-tetradecadienoate and 3,4-tetradecadienoic acid are online here.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
