Mass Spectra of Fatty Alcohols: 1. Nicotinate Derivatives
Fatty alcohols can be characterized by GC-MS most comprehensively as the nicotinate derivatives, first described by Vetter and Meister (1981). These are structurally analogous to 3‑pyridylcarbinol ('picolinyl') esters and fragment in much the same way. Indeed, with alkyl moieties of the same basic structure, nicotinates and 3-pyridylcarbinol esters have identical molecular weights. The two most abundant ions at m/z = 107 and 124 correspond to fragmentation at the carboxyl moiety, illustrated simplistically here, and thereafter, interpretation of spectra is comparable to that for the analogous 3‑pyridylcarbinol esters. Nicotinates are also useful for structural determination of diacylglycerols and other lipids with a free hydroxyl group (Dobson, G. et al, 1998).
D.J. Harvey was responsible for much of the early work on nicotinates, and his review article is most valuable (Harvey, 1992). The mass spectra of the nicotinates of fatty alcohols illustrated here were obtained in a study of fish waxes (Joh et al., 1995), and the preparation procedure used in our laboratory is described at the end of this document. Part 2 of this document deals with trimethylsilyl ethers and other derivatives of fatty alcohols.
Saturated and Branched-Chain Fatty Alcohols
The mass spectrum of the nicotinate derivative of hexadecan-1-ol is illustrated first -
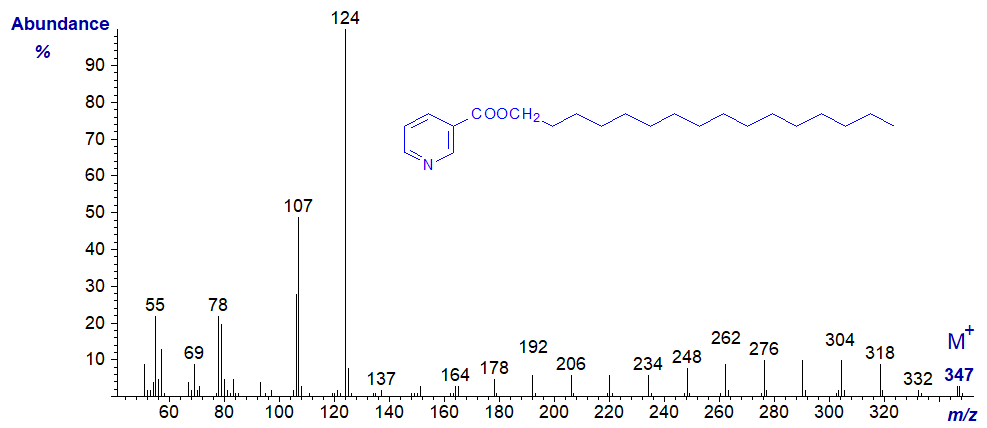
As with 3-pyridylcarbinol esters, interpretation is easiest if we start with the molecular ion and work backwards (see the web page on 3‑Pyridylcarbinol esters of saturated fatty acids). There is a gap of 15 amu for loss of the terminal methyl group to m/z = 332 and then a series of ions 14 amu apart for fragmentations at successive methylene groups until the characteristic ions at m/z = 124 and 107 are reached.
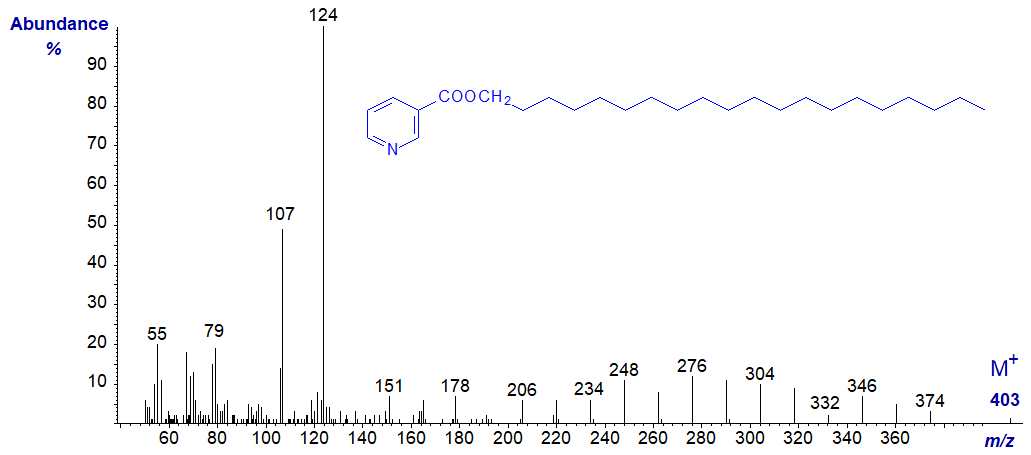
The same features are seen in the mass spectrum of the nicotinate derivative of eicosan-1-ol next -
Branched fatty alcohols can be identified by this means in a similar manner to 3-pyridylcarbinol esters, as in the spectrum of the nicotinate derivative of 14‑methyl-hexadecanol (anteiso-isomer) -
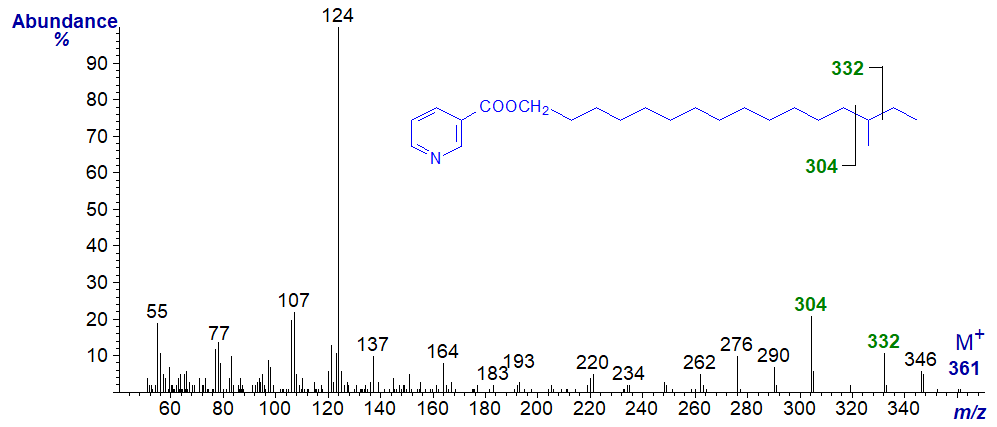
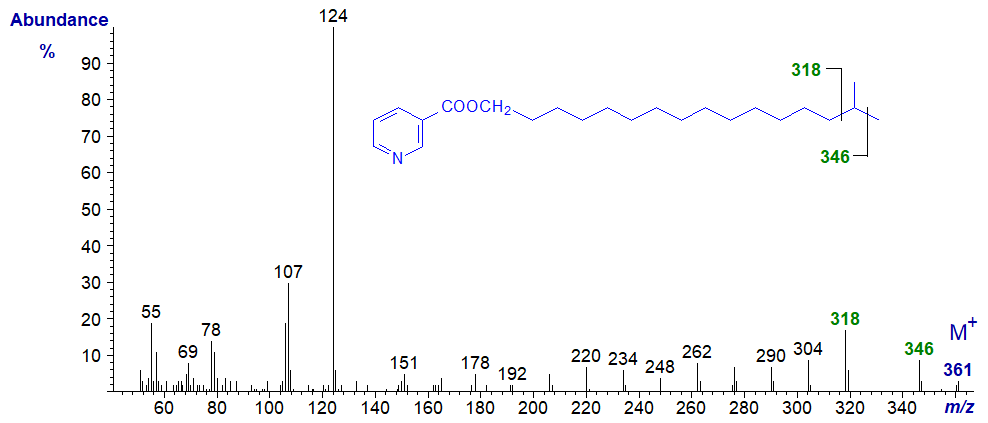
The gap of 28 amu between m/z = 304 and 332 reflects cleavage on either side of the carbon carrying the methyl group. As might be expected, this gap is shifted to between m/z = 318 and 346 in the spectrum of the nicotinate derivative of the iso-isomer (15-methyl-hexadecanol) next -
Monoenoic Fatty Alcohols
The mass spectrum of the nicotinate derivative of octadec-9-en-1-ol is -
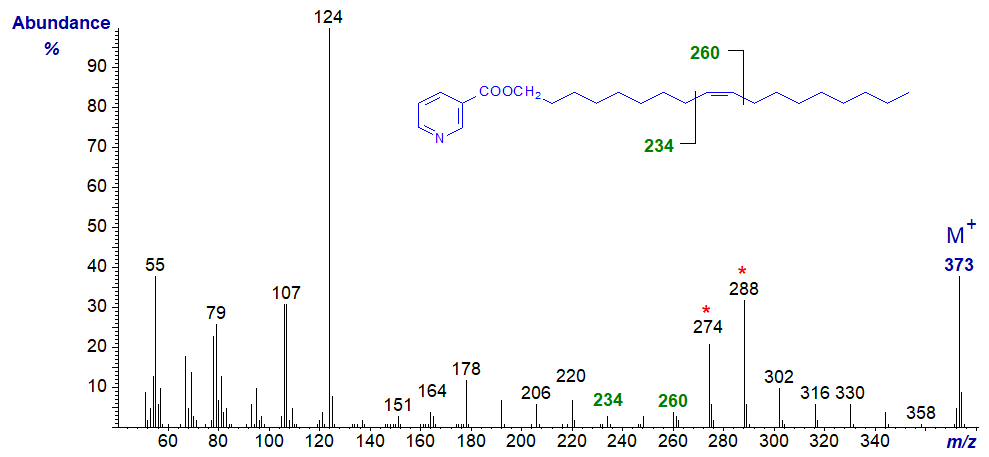
Again, the spectrum resembles that for a 3-pyridylcarbinol ester (see the web pages on 3-Pyridylcarbinol esters of monoenoic fatty acids). The double bond is located by the gap of 26 amu between m/z = 234 and 260, while the doublet of ions at m/z = 274 and 288 is an invaluable signpost (identical ions are present in the spectrum of 3-pyridylcarbinyl oleate).
The mass spectrum of the nicotinate derivative of octadec-11-en-1-ol -
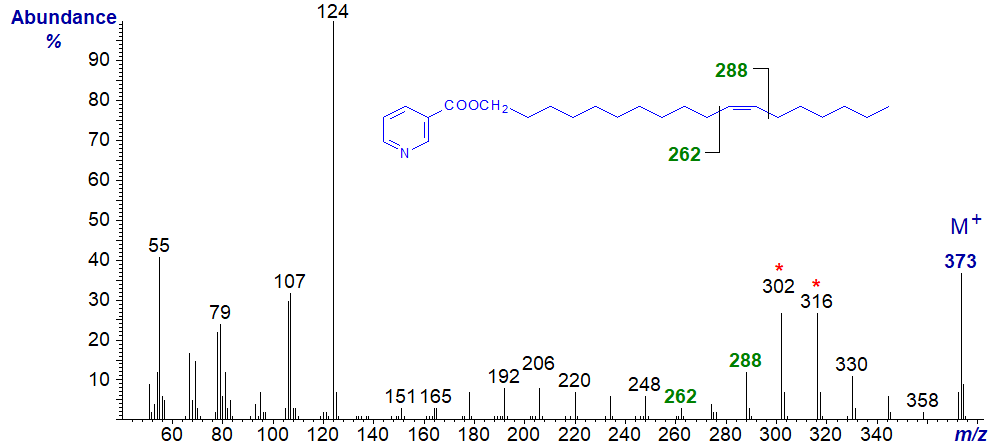
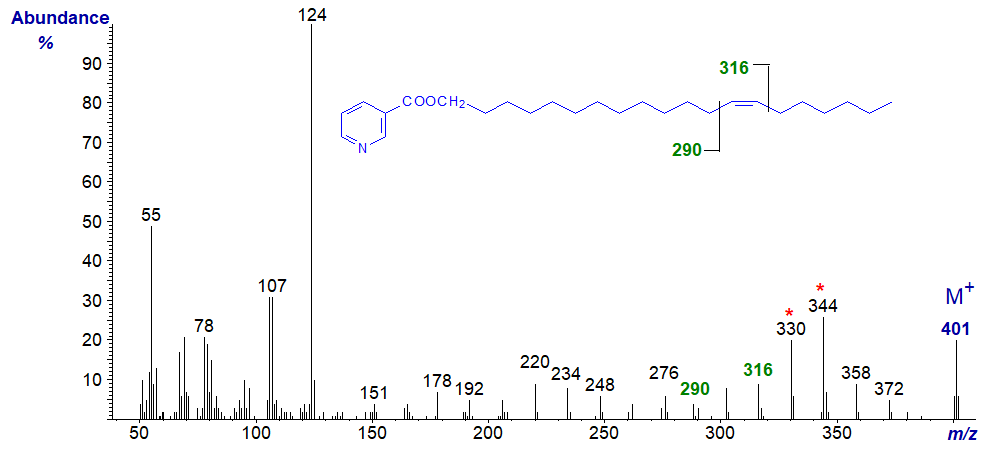
Here, the diagnostic ions are shifted upwards by 28 amu, i.e., for the double bond to between m/z = 262 and 288, with the doublet at m/z = 392 and 316. A further shift upwards of 28 amu for the diagnostic ions is then seen in the spectrum of the nicotinate of eicos-13-en‑1‑ol -
Di- and Polyenoic Fatty Alcohols
The spectra reported here were from the first definitive finding of such fatty alcohols in a fish oil (Joh et al. (1995)). The mass spectrum of the nicotinate derivative of octadeca-9,12-dien-1-ol (the analogue of linoleic acid) is -
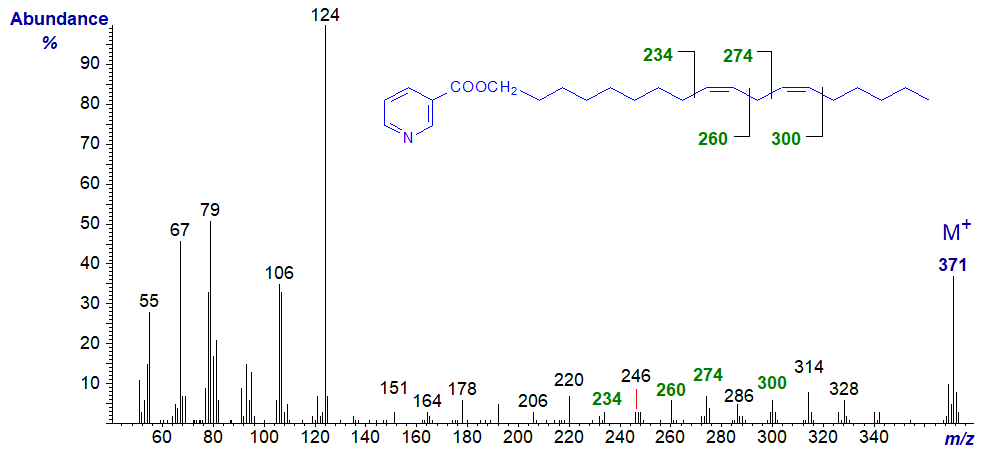
The double bonds in positions 9 and 12 can be located by the gaps of 26 amu between m/z = 234 and 260, and 274 and 300, respectively. On the other hand, the limited information available suggests that a gap of 12 amu, analogous to that found with DMOX derivatives, may be more useful with nicotinates, i.e., from m/z = 234 to 246, and 274 to 286, respectively.
The mass spectrum of the nicotinate derivative of octadeca-11,14-dien-1-ol -
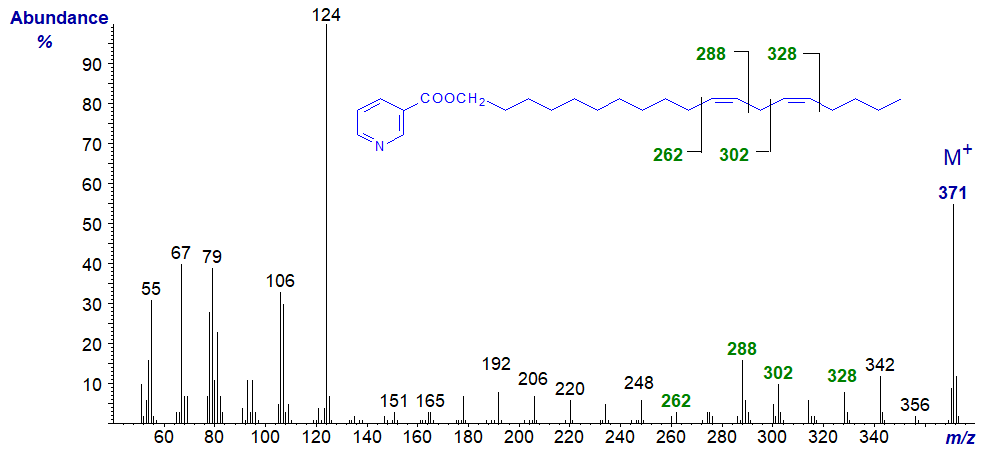
In this instance, the diagnostic ions are again shifted upwards by 28 amu from the previous spectrum.
The mass spectrum of the nicotinate derivative of eicos-5,8,11,14,17-pentaen-1-ol -
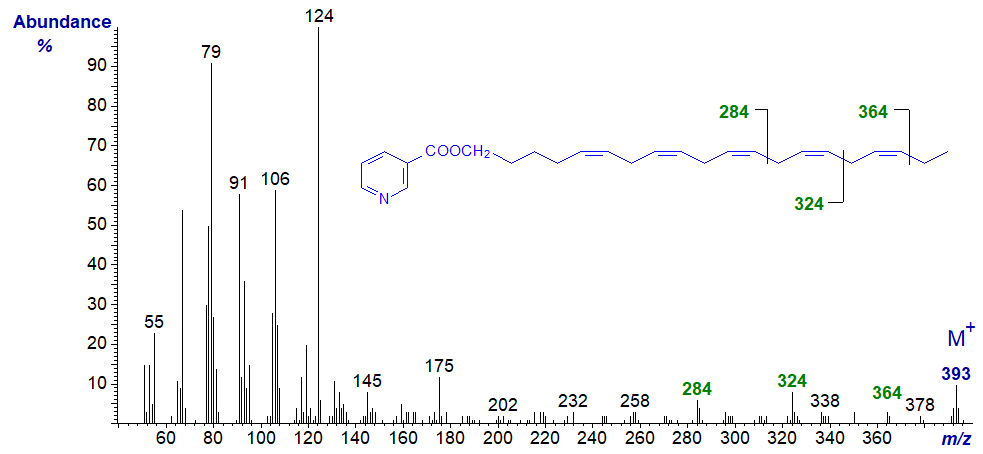
Here, only the terminal double bonds can be located with confidence, and these by the gaps of 40 amu for the methylene groups followed by the double bond, i.e., from m/z = 284 to 324, and 324 to 364, for the double bonds in positions 14 and 17 respectively.
The mass spectrum of the nicotinate derivative of docos-7,10,13,16,19-pentaen-1-ol -
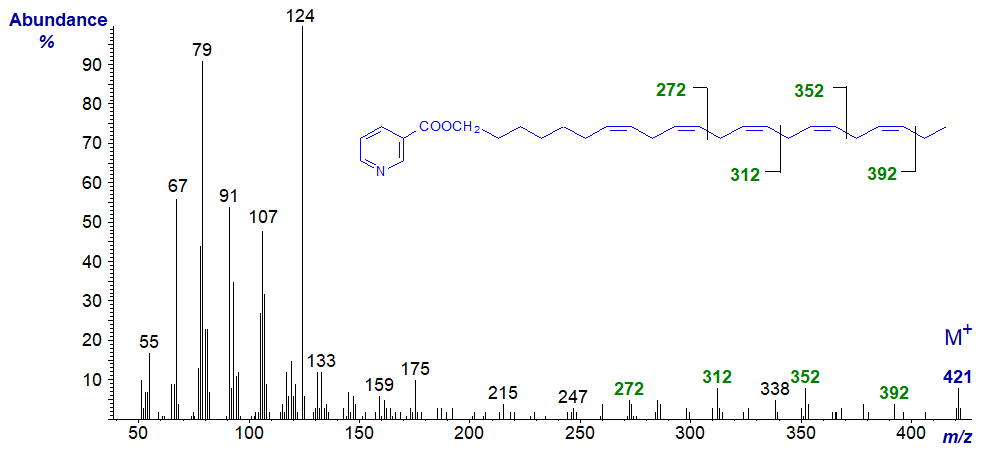
Again, the double bonds in positions 13, 16 and 19 only can be located from the gaps of 40 amu between m/z = 272 and 312, 312 and 352, and 352 and 392, respectively.
There has been a suggestion in the literature that rather than preparing 3-pyridylcarbinol esters of fatty acids for structural analysis, better results might be obtained by reducing them to the aliphatic alcohols and then preparing nicotinates for mass spectrometry. From my admittedly limited experience of nicotinates relative to 3‑pyridylcarbinol esters, I have to disagree.
Alkan-2-ols
From the limited number of mass spectra available to us, those of nicotinates of alkan-2-ols are almost indistinguishable in terms of which ions are formed from those of the corresponding alkan‑1‑ols. As an example, the mass spectrum of the nicotinate of hexadecan-2-ol is illustrated next -
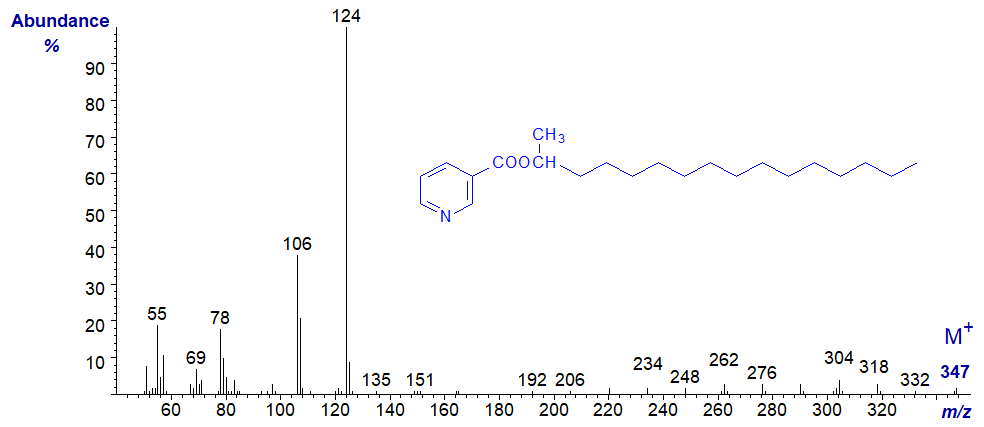
Essentially the same ions are present in the spectra of both isomers, but their relative abundances in the spectrum of the 2-alkanol derivative are lower in the high mass range. While the ion at m/z = 106 is more abundant than that at m/z = 107, we do not have sufficient spectra to be sure that this is a consistent feature. In contrast, 1- and 2-alkanols are readily distinguishable as the trimethylsilyl ether derivatives (see the web page dealing with TMS derivatives of alkanols), although double bond positions cannot be determined with the latter.
We have some spectra of further nicotinate derivatives of fatty alcohols on file in our Archive pages, but without interpretation.
Preparation of Nicotinates
The following method has been recommended for the preparation of the nicotinate derivatives (Dobson et al. 1998) and was adapted from a procedure by Zollner and Schmid (1996).
| Laboratory Protocol: At 0°C, N,N'-dicyclohexylcarbodiimide (Caution - carcinogenic!) (20 mg) is added to a solution of fatty alcohols (up to 2 mg), nicotinic acid (10 mg) and 4-dimethylaminopyridine (2 mg) in dichloromethane (3 mL). After 5 minutes, the mixture is allowed to warm to room temperature and then left overnight. Hexane (2 mL) is then added, and the product filtered through a cotton wool plug pre-washed with hexane. After taking to dryness, the product is purified by solid-phase extraction through a bonded NH2 column. After pre-washing the column with hexane, the product is applied in hexane and the column is first washed with hexane (8 mL). The nicotinyl esters are recovered by elution with hexane-acetone (95:5, v/v; 10 mL). |
It is also possible to prepare nicotinates by reaction of fatty alcohols with nicotinyl chloride in the presence of pyridine (Joh et al., 1995).
References and Suggested Reading
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Dobson, G., Itabashi, Y., Christie, W.W. and Robertson, G.W. Liquid chromatography with particle-beam electron-impact mass spectrometry of diacylglycerol nicotinates. Chem. Phys. Lipids, 97, 27-39 (1998); DOI.
- Harvey, D.J. Mass spectrometry of picolinyl and other nitrogen-containing derivatives of lipids. In: 'Advances in Lipid Methodology - One', pp. 19-80 (edited by W.W. Christie, Oily Press, Ayr) (1992).
- Harvey, D.J. and Tiffany, J.M. Comparison of derivatives for the characterization of branched long-chain alcohols and 1,2-diols by mass spectrometry. Biomed. Mass Spectrom., 11, 353-359 (1984); DOI.
- Joh, Y.-G., Brechany, E.Y. and Christie, W.W. Characterization of wax esters in the roe oil of the amber fish Seriola aureovittata, by silver ion high-performance liquid chromatography. J. Am. Oil Chem. Soc., 72, 707-713 (1995); DOI.
- Vetter, W. and Meister, W. Nicotinates as derivatives for the mass spectrometry investigation of long chain alcohols. Org. Mass Spectrom., 16, 118-122 (1981); DOI.
- Zollner, P. and Schmid, R. Utility of nicotinoyl derivatives in structural studies of mono- and diacylglycerols by GC/MS. Part 3. Application to acylglycerols with methyl branchings and epoxy and cyclopropyl rings. J. Mass Spectrom., 31, 411-417 (1996); DOI.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
