Mass Spectrometry of Fatty Alcohols:
2. Derivatives other than Nicotinates
 While most
structural information is obtainable from fatty alcohols as the nicotinate derivatives,
there are many circumstances when this is not practicable or necessary.
It is relevant that nicotinates have much higher molecular weights, so need high temperatures or non-polar phases for GC analysis.
When alkanol samples are reasonably familiar, the simplest approach is to analyse them in the underivatized state
or better as trimethylsilyl (TMS) ethers.
t-Butyldimethylsilyl ethers are more stable chemically and have been widely used for analysis, but we have no spectra available
to discuss here.
Acetates and isopropylidene derivatives (for diols) still have some applications.
While most
structural information is obtainable from fatty alcohols as the nicotinate derivatives,
there are many circumstances when this is not practicable or necessary.
It is relevant that nicotinates have much higher molecular weights, so need high temperatures or non-polar phases for GC analysis.
When alkanol samples are reasonably familiar, the simplest approach is to analyse them in the underivatized state
or better as trimethylsilyl (TMS) ethers.
t-Butyldimethylsilyl ethers are more stable chemically and have been widely used for analysis, but we have no spectra available
to discuss here.
Acetates and isopropylidene derivatives (for diols) still have some applications.
As an example, waxes contain many different types of aliphatic constituents, and the best means of characterizing the complex mixtures can often be to hydrolyse and then silylate the whole product for analysis by GC-MS. Fatty alcohols in such samples tend to have relatively simple compositions, being mainly saturated or monoenoic, with occasional iso- and anteiso-methyl branches, especially in waxes, so sufficient information may be obtainable by this means to identify a high proportion of the components with reasonable certainty. Access to GC retention data is a further aid to identification. As with other documents on this website, only those spectra encountered in our own research work can be illustrated.
Practical procedures for preparing the various derivatives discussed here are set out at the end of this web page. No references are cited here for specific mass spectra as establishing priority from the early literature is now difficult, and most of the above mass spectra will not have been published formally elsewhere. I am not aware of a suitable review article that I can recommend, but the reading list at the end of the page should prove useful.
Underivatized Fatty Alcohols
Mass spectra of underivatized primary fatty alcohols contain relatively little structural information, and the mass spectrum of hexadecan-1-ol is -
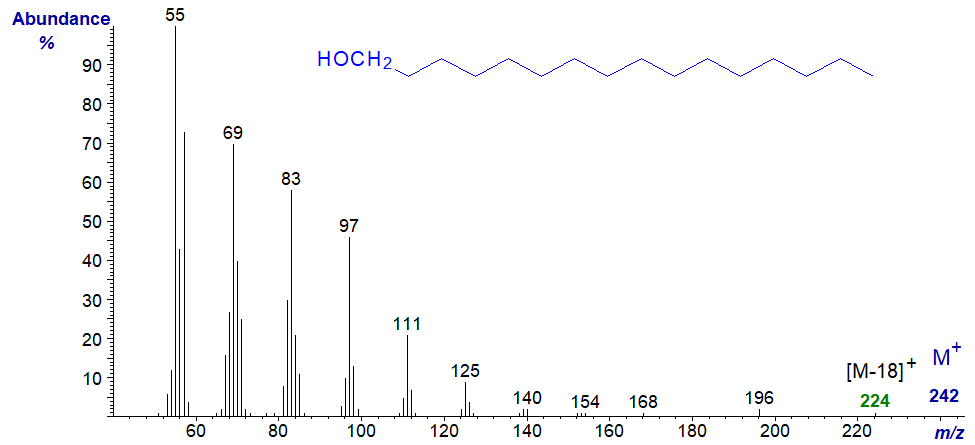
The molecular ion is rarely seen without substantial magnification, and the first significant if small ion in the high mass region is that representing the loss of the elements of water ([M‑18]+), in this instance at m/z = 224. The remaining ions are hydrocarbon fragments. The ions at m/z = 196 and 168 are characteristic and are presumed to represent loss of successive C2 units.
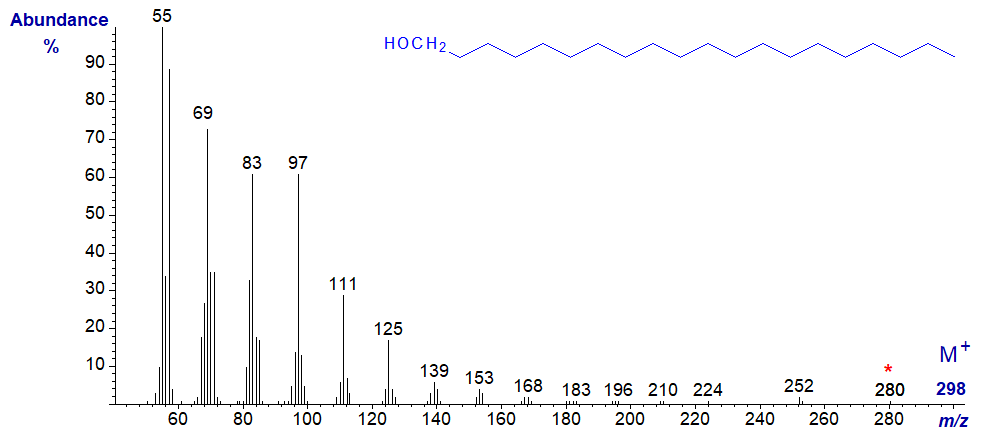
Essentially the same features are seen in the mass spectrum of eicosan-1-ol next -
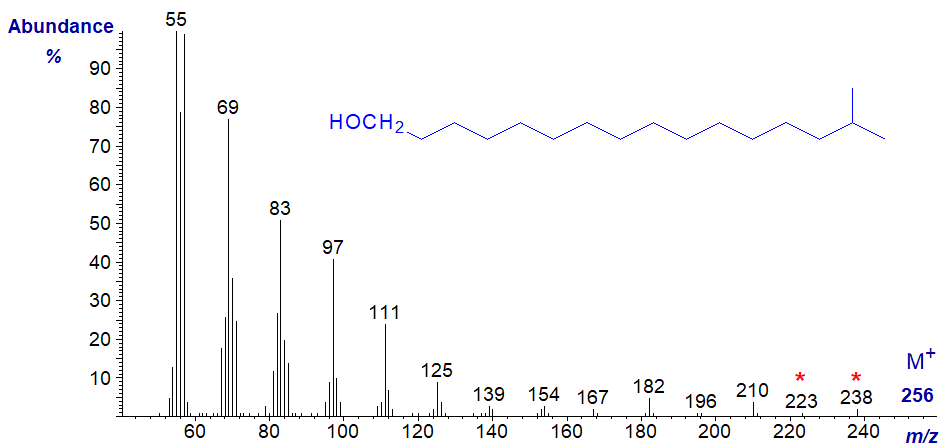
The mass spectrum of 15-methyl-hexadecan-1-ol, i.e., with an iso-methyl group, is likewise undistinguished, although an ion at m/z = 223, which may represent loss of one of the terminal methyl groups following the loss of water could be diagnostic, as are ions representing loss of successive methylene groups (at m/z = 210, 196, 182, etc.). It would be necessary to have access to spectra of more isomers to be sure of this.
The mass spectra of monoenoic primary alcohols are comparable to the saturated equivalents, except that many of the main ions are 1-2 amu lower than in the corresponding saturated alcohol. This can be seen from the spectrum of hexadec-9-en-1-ol next -
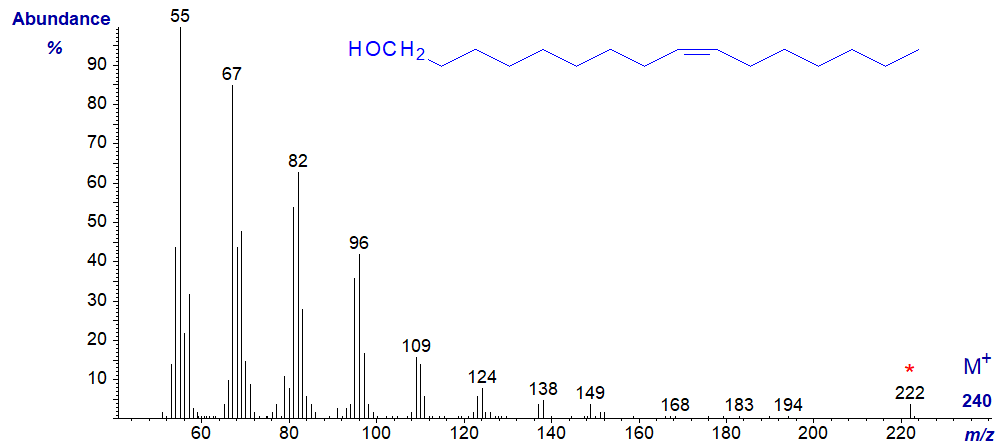
Further spectra are available in the Archive section of this site, but without interpretation.
Acetate Derivatives of Alcohols
As the molecular ion is absent for all practical purposes, the first significant ion in the high mass region of the mass spectrum of an acetate derivative is that for loss of acetic acid ([M-60]+). In the spectrum of 1-tetracosanyl acetate, illustrated below, this is at m/z = 336. A distinctive ion at m/z = 61 in the spectra of acetates is presumed to be a protonated acetate moiety - [CH3COOH2]+. Little further useful information can be obtained from the spectrum. 1- and 2‑Alkanol acetates can be distinguished by mass spectrometry, as ions at m/z = 73 and 116 occur in 1-acetates only, while ions at m/z = 87 and 102 are found only in 2‑acetates (Naccarato, W.F. et al., 1972). I have no spectra of the latter for illustrative purposes.
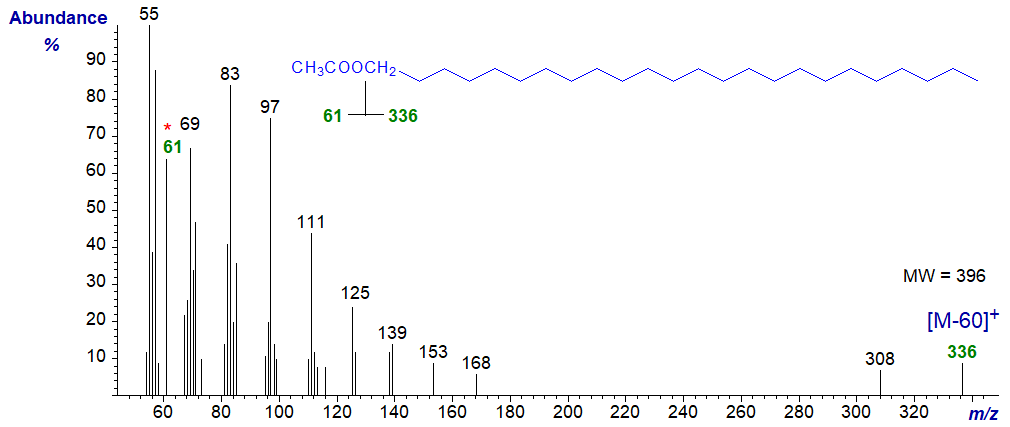
It should be noted that the base ion in the spectra of acetates is often at m/z = 43 (or 44), representing the [CH3CO]+ moiety, but ions below m/z = 50 were automatically edited out by our software when the spectra illustrated here were prepared.
The mass spectrum of 1-octadec-9,12-dienyl acetate –
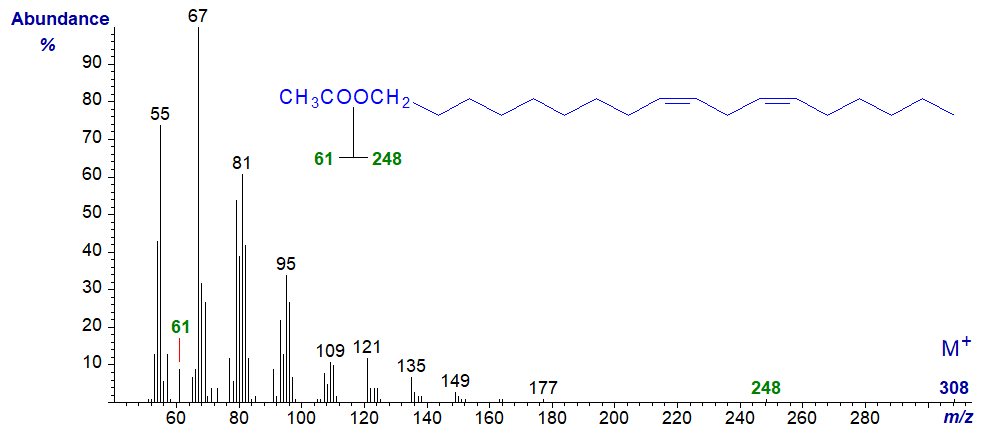
In this instance, the molecular ion is now discernible, together with the ion for [M–60]+ at m/z = 248, although that at m/z = 61 is smaller than in the spectra of saturated analogues.
Trimethylsilyl Ether Derivatives of Alcohols
Trimethylsilyl (TMS) ether derivatives are probably used more widely than any other for the gas chromatographic analysis of hydroxy compounds in general and for aliphatic alcohols as described here. Their main value lies in increasing the volatility and reducing the polarity of the parent molecules, so ensuring sharp symmetrical peaks on GC analysis. On the other hand, the mass spectrometric properties of the saturated primary alcohols are not exceptional, although they usually do permit the molecular weight to be determined at least, even if the molecular ion per se is small and often not detectable. Tulloch, A.P. (1972) has described mechanistic aspects of their mass spectra.
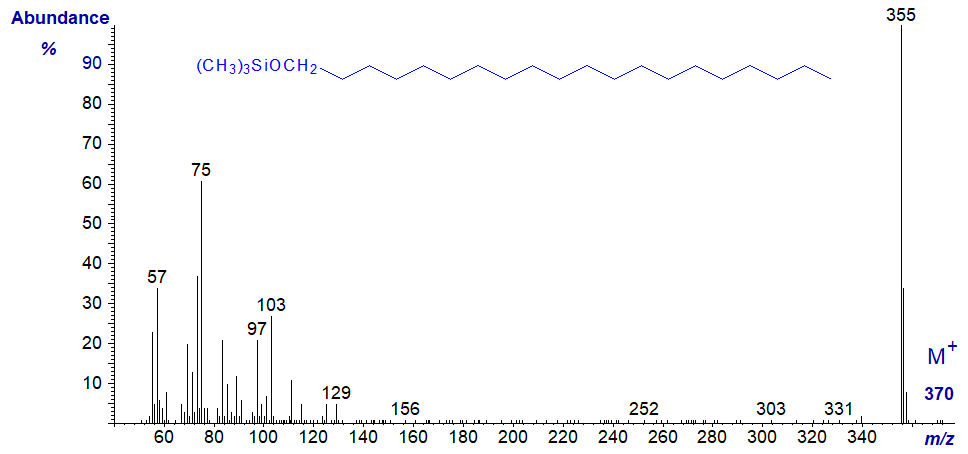
In the mass spectrum of the TMS ether of eicosan-1-ol, the base ion (m/z = 355) represents the loss of a methyl group from the TMS ether moiety. It is a characteristic fingerprint at least.
Mass spectra of the TMS ethers of unsaturated alk-1-ols are rather different, and that of the TMS ether of eicos-11-en-1-ol is illustrated –
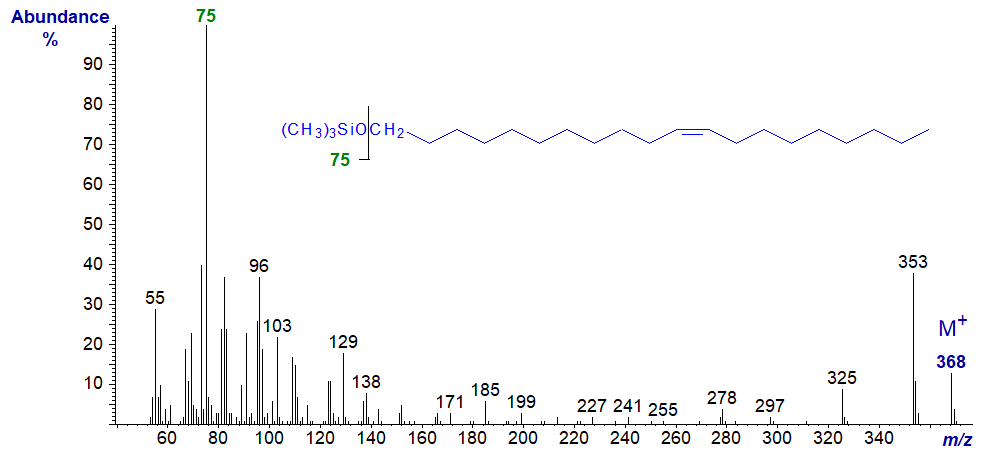
In this instance, there is an adequate molecular ion, and the base ion at m/z = 75 represents the trimethylsilyloxy group. There are no ions that serve to locate the double bond of course. Further spectra of TMS ether derivatives of aliphatic primary alcohols are available in our Archive pages, but without interpretation.
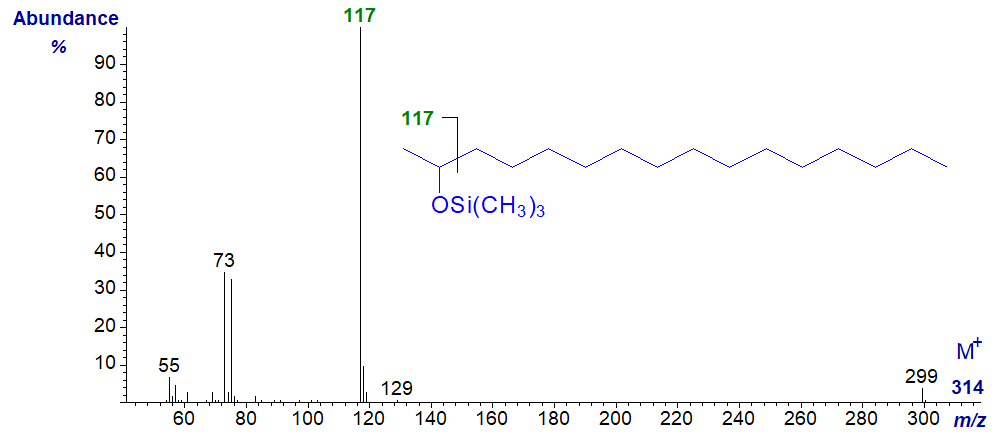
The mass spectrum of the trimethylsilyl derivatives of a secondary alcohol is very different from that of a primary alcohol, and that of the TMS derivative of hexadecan-2-ol is illustrated. In this instance the base ion at m/z = 117 represents cleavage alpha to the carbon carrying the hydroxyl moiety.
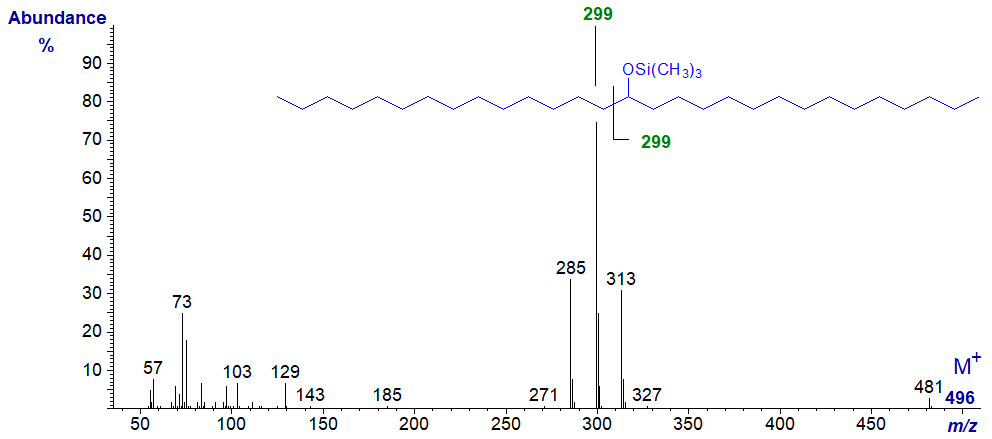
When the OTMS group is more centrally located, the main cleavage is again alpha to the carbon carrying the hydroxyl moiety (m/z = 299), as in the spectrum of the TMS ether of 15-hydroxy-nonacosane (courtesy of Isabel Molina, Mike Pollard and John Ohlrogge) -
Aliphatic Diols
Aliphatic 1,2-diols are occasional components of waxes, and the spectra recorded here were obtained from constituents of beeswax. Isopropylidene derivatives are useful for characterization purposes in general, as they are only formed from compounds with hydroxyl groups on adjacent carbon atoms, but they do not give mass spectra that are very informative. The mass spectrum of the isopropylidene derivative of hexadecane-1,2-diol is -
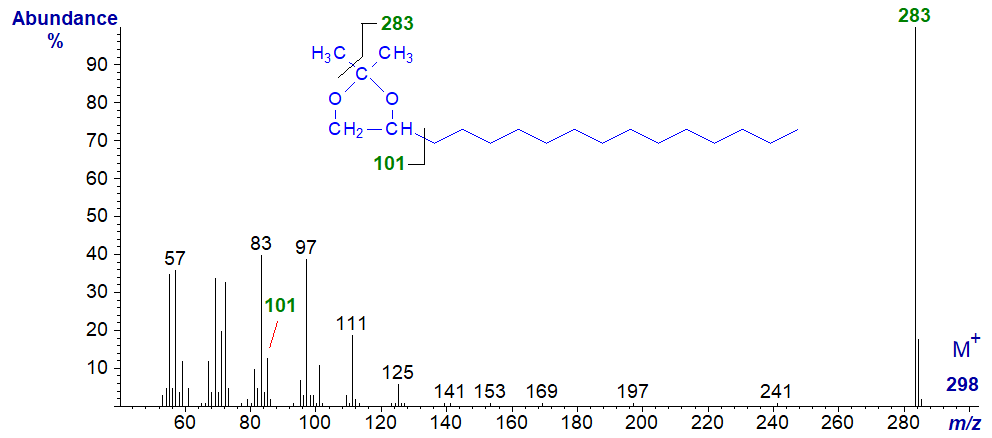
The molecular ion is essentially absent, but the base ion represents loss of one of the methyl groups from the isopropylidene moiety, at m/z = 283 in this instance. This enables precise determination of molecular weight, always an important parameter. The ion at m/z = 101 confirms that the diol moiety is in the 1,2-position, although this is hardly necessary as 1,3-diols would not form isopropylidene derivatives
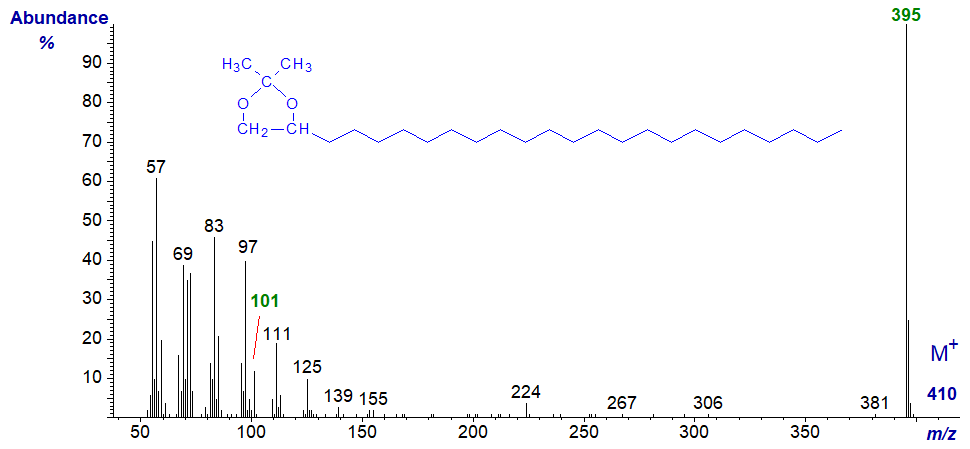
The mass spectrum of the isopropylidene derivative of tetracosane-1,2-diol, which follows, is entirely analogous to this.
Mass spectra of bis-TMS ethers of 1,2-aliphatic diols tend to be only a little more informative, and as an example, the mass spectrum of the bis-TMS ether of hexadecane-1,2-diol follows -
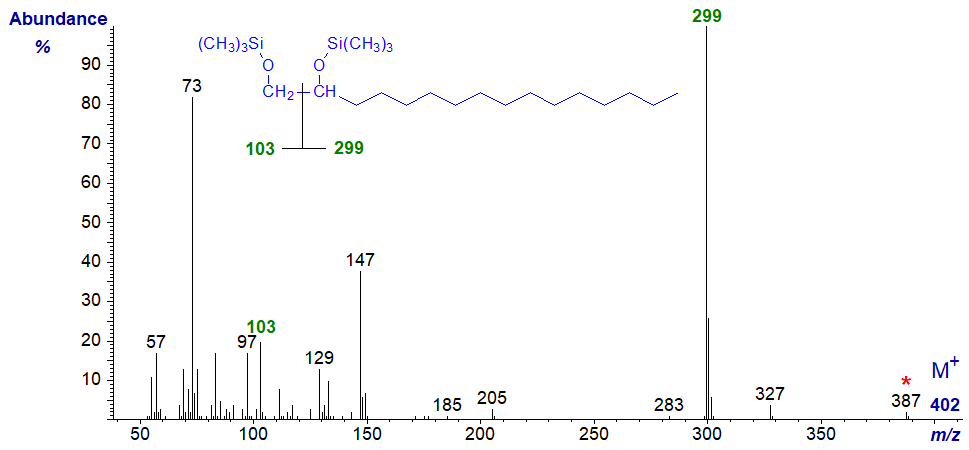
The molecular ion is not apparent, but an ion at m/z = 387 for loss of a methyl group enables determination of the molecular weight. The main cleavage is at the centre of the diol system yielding two ions at m/z = 103 and 299 (the base ion) that clearly define the structure of the molecule.
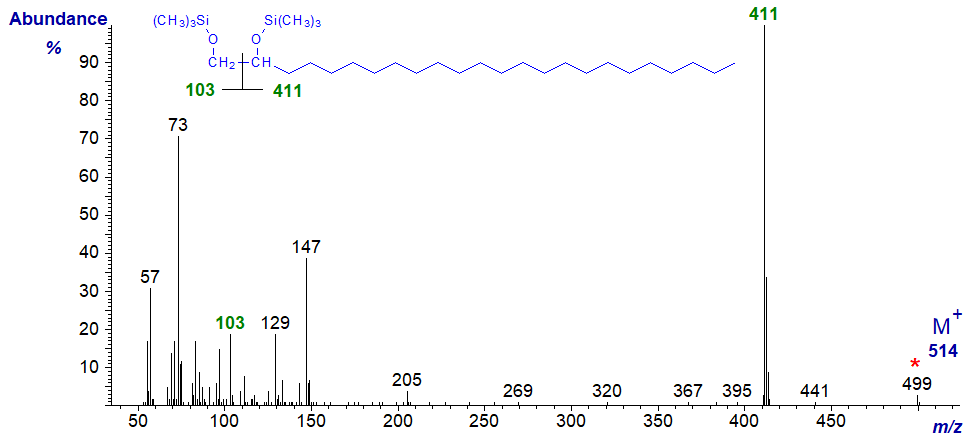
With the mass spectrum of the bis-TMS ether of tetracosane-1,2-diol -
α,ω-Aliphatic diols occur in plant cutins, but the mass spectra of their TMS ethers have limited value. That of the TMS ether of 1,16‑hexadecanediol is illustrated for record purposes -
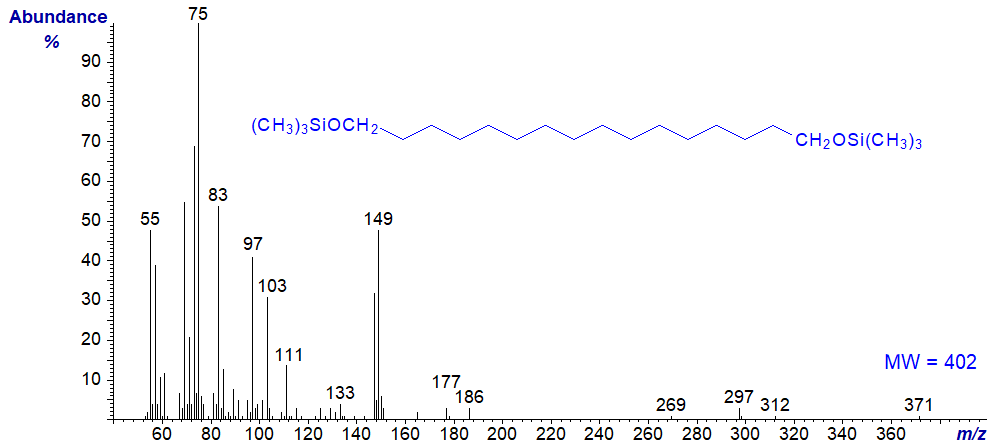
Presumably, the ion at m/z = 371 represents loss of methyl groups from both TMS groups, while that at m/z = 149 is not easy to interpret.
Further spectra of aliphatic diols in the form of both types of derivative are available in the Archive section of this site, but without interpretation. These include several saturated isomers with iso- and anteiso-methyl branches, but I am unable to find any features in their mass spectra that distinguish these.
Preparation of Derivatives
The following methods are adapted from Christie and Han (2010). Several reagents are available for trimethylsilylation of which bis(trimethylsilyl)acetamide ('BSA') is most popular. As TMS esters are very sensitive to traces of moisture, most analysts simply inject an aliquot of the reaction mixture directly onto the GC column, though this is only suitable with non-polar stationary phases.
| Trimethylsilylation - laboratory protocol: The reaction is carried out simply by dissolving the lipid in a solvent such as acetone or acetonitrile and adding a ten-fold excess by weight of BSA. With the normal range of unhindered alcohols likely to be encountered, the reaction is complete in 10 minutes at room temperature, though longer reaction times may be necessary for some sterols. Finally, the sample is diluted with isohexane to a concentration appropriate for GC analysis. |
The following method is suitable for the preparation of isopropylidene derivatives.
| Isopropylidene derivatives - laboratory protocol: The lipids (10 mg) are dissolved in dry acetone (3 mL) and anhydrous copper sulfate (50 mg) is added. After 24 hr. at room temperature (or 3 hr. at 50ºC), the solution is filtered, the copper salts are washed with dry diethyl ether and the combined solutions are evaporated in a gentle stream of nitrogen. |
Acetylation is equally straightforward.
| Acetylation - laboratory protocol: The lipid (up to 50 mg) is dissolved in acetic anhydride in pyridine (2 mL, 5:1. v/v) and is left at room temperature overnight. The reagents are then removed in a stream of nitrogen with gentle warming and the acetylated lipid can be purified, if necessary, by preparative TLC on silica gel layers with hexane-diethyl ether (80:20, v/v) as the mobile phase. |
References - Suggested Reading
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Harvey, D.J. and Tiffany, J.M. Comparison of derivatives for the characterization of branched long-chain alcohols and 1,2-diols by mass spectrometry. Biomed. Mass Spectrom., 11, 353-359 (1984); DOI.
- Murphy, R.C. Mass Spectrometry of Lipids (Handbook of Lipid Research, Vol. 7) (Plenum Press, N.Y.) (1993).
- Naccarato,W.F., Gelaman,R.A., Kawalek,J.C. and Gilbertson,J.R. Characterisation and metabolism of free fatty alcohols from E. coli. Lipids, 7, 275-281 (1972); DOI.
- Tulloch, A.P. Mass spectra of TMS esters of deuterated decanoic acids and TMS ethers of deuterated decanols. Lipids, 20, 404–411 (1985); DOI.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
