Mass Spectrometry of Methyl Esters
Trienoic Fatty Acids
As cautioned in the Introduction to these documents, mass spectra of methyl esters obtained under electron-impact ionization afford limited information only concerning the double bond positions in fatty acids. The molecular weight is usually obtainable, and this is an important piece of information. If chromatographic retention data are added to this, it is often possible to be reasonably sure of the identity of a fatty acid. In contrast to the situation with monoenes and dienes, there are a few key ions that help to identify the common polyenoic fatty acids with methylene-interrupted ('homo-allylic') unsaturation and importantly those of the (n‑6) and (n‑3) families, though the presence of these ions must be interpreted with caution. Some other polyunsaturated fatty acids in the form of methyl esters give distinctive spectra, which are useful as fingerprints for identification purposes, although the mechanisms of fragmentation may not always be properly understood. In our Archive, mass spectra of ten natural 18:3 isomers differing in double bond positions (not counting Z/E isomers) are illustrated, all of which appear to be distinctive and characteristic. A few of the spectra illustrated below may have been published elsewhere (references cited if known to me), but many are illustrated here for the first time.
Methylene-Interrupted Trienes
In the mass spectra of methyl esters of methylene-interrupted trienes, as opposed to tetraenes to hexaenes, there is usually a distinctive molecular ion together with a small ion at [M−31/32]+ for loss of the elements of a methoxyl group (plus a hydrogen atom). The McLafferty ion (m/z = 74) is always small. In the lower molecular weight region, hydrocarbon ions of general formula [CnH2n‑5]+ tend to dominate the spectrum with the ion at m/z = 79 as the base ion followed by m/z = 93, 107, 121, 135 and so forth.
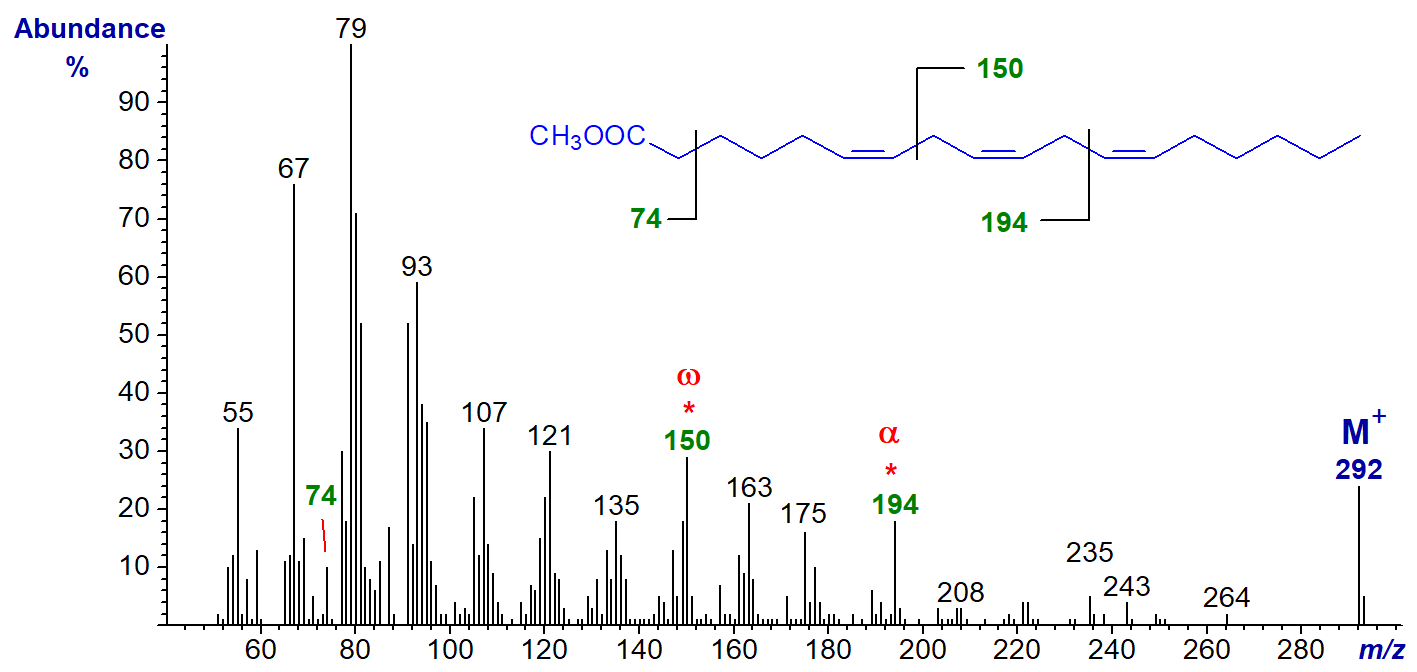
There are two relatively common C18 trienoic acids and their mass spectra are illustrated below, starting with methyl 6,9,12‑octadecatrienoate (γ‑linolenate or 18:3(n‑6)) (Holman and Rahm, 1971) -
- and of methyl 9,12,15-octadecatrienoate (α-linolenate or 18:3(n-3)) (Hallgren et al., 1959)-
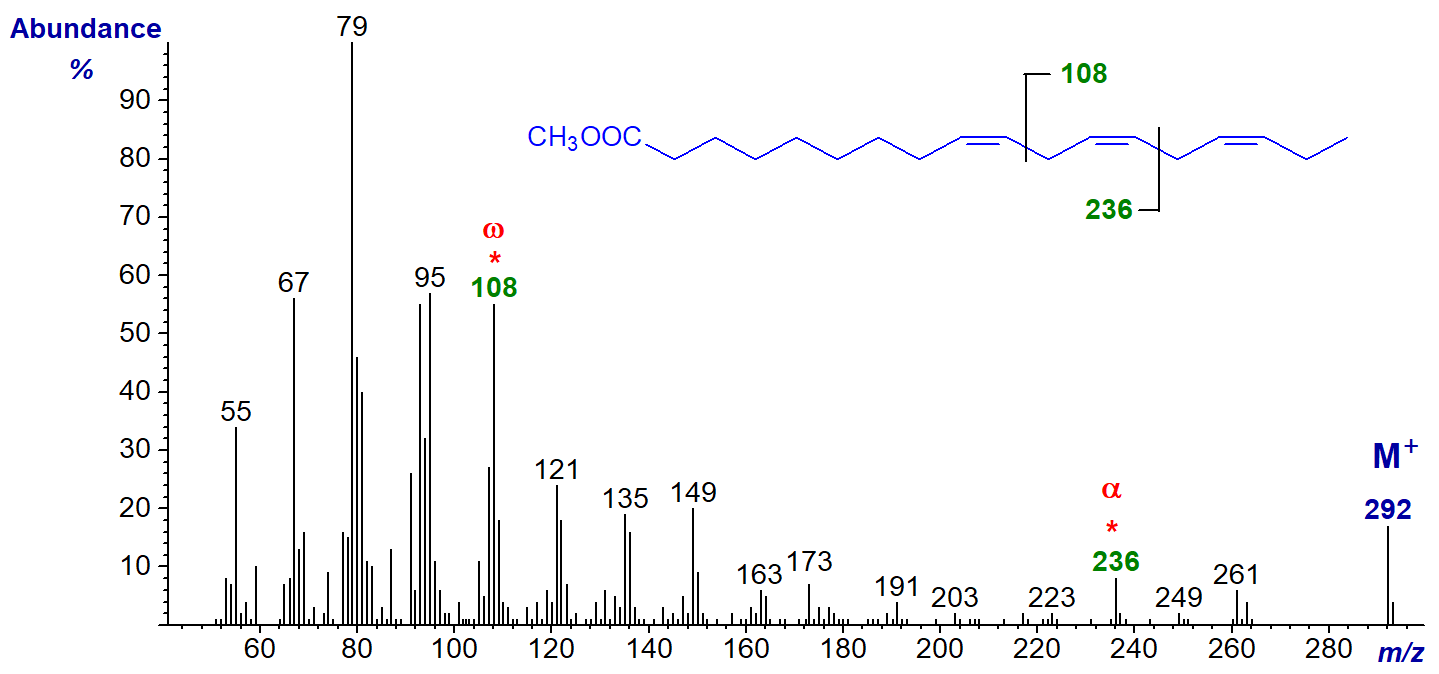
Both spectra are superficially similar, but there are features that make them something more than simply ‘fingerprints’ for identification purposes. For example, an ion at m/z = 150 is characteristic for methyl esters of polyunsaturated fatty acids with an n‑6 terminal double bond, while one at m/z = 108 defines an n‑3 terminal double bond, assuming methylene-interrupted unsaturation (Holman and Rahm, 1971; Brauner et al.,1982; Fellenberg et al., 1987)). For the minor (n-9) and (n-4) families the relevant ions are at m/z = 192 and 122, respectively. Although such key ions always result from complex rearrangements, they are illustrated simplistically for practical purposes by cleavages in the positions shown.
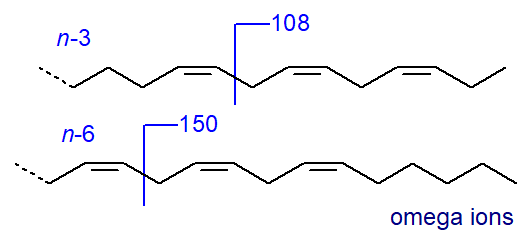 |
| Figure 3. Mass spectral fragmentations for omega-6 and omega-3 esters - omega ions. |
My impression is that each of these ions occurs with reasonable consistency in the spectra of fatty acid methyl esters from these biochemical families, although they are not necessarily unique to such families. It should be noted that these ions, which are sometimes termed the ‘omega’ (ω) ions, are relevant only for fatty acids with three or more methylene-interrupted double bonds and not for dienes.
In addition, there are small ions formed by a comparable cleavage at the carboxyl end of the molecule giving a fragment containing the first two double bonds and the second methylene group (minus a proton) that could be termed the 'alpha' (α) ion, as illustrated. Thus, in the mass spectrum of methyl 6,9,12‑octadecatrienoate, this ion is at m/z = 194, and it appears to be present in the spectra of all conventional methylene-interrupted polyenoic acids to which we have access with the first double bond in position 6. The corresponding ion in the spectrum of methyl 9,12,15-octadecatrienoate is at m/z = 236. This ion was first noted by Holman and Rahm (1971), but it was studied more systematically via chemical ionization methods in a paper by others that appears to have been largely overlooked (Brauner et al., 1982).
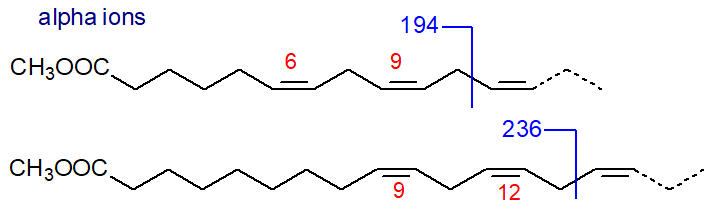 |
| Figure 4. Mass spectral fragmentations at the carboxyl end - alpha ions. |
Analogous ions are seen in spectra of methyl esters of most methylene-interrupted polyunsaturated fatty acids (three or more double bonds) as listed in Table 1. The alpha ions are not always as easily distinguished as the omega ions, but they do appear always to be present other than in some of the fatty acids of the lesser known (n-1) family) (author, unpublished observation).
Table 1. Alpha (α) ions in the mass spectra of methyl esters of polyunsaturated fatty acids. |
||||||||
| First double bonds | Δ4,7 | Δ5,8 | Δ6,9 | Δ7,10 | Δ8,11 | Δ9,12 | Δ10,13 | Δ11,14 |
| Ion (m/z) | 166 | 180 | 194 | 208 | 222 | 236 | 250 | 264 |
With the biologically important C20 analogues, such as 8,11,14-eicosatrienoate (20:3(n-6)) and the mass spectrum of the methyl ester, the omega ion at m/z = 150 is again prominent together with the alpha ion for a Δ8,11 double bond system at m/z = 222 in this instance -
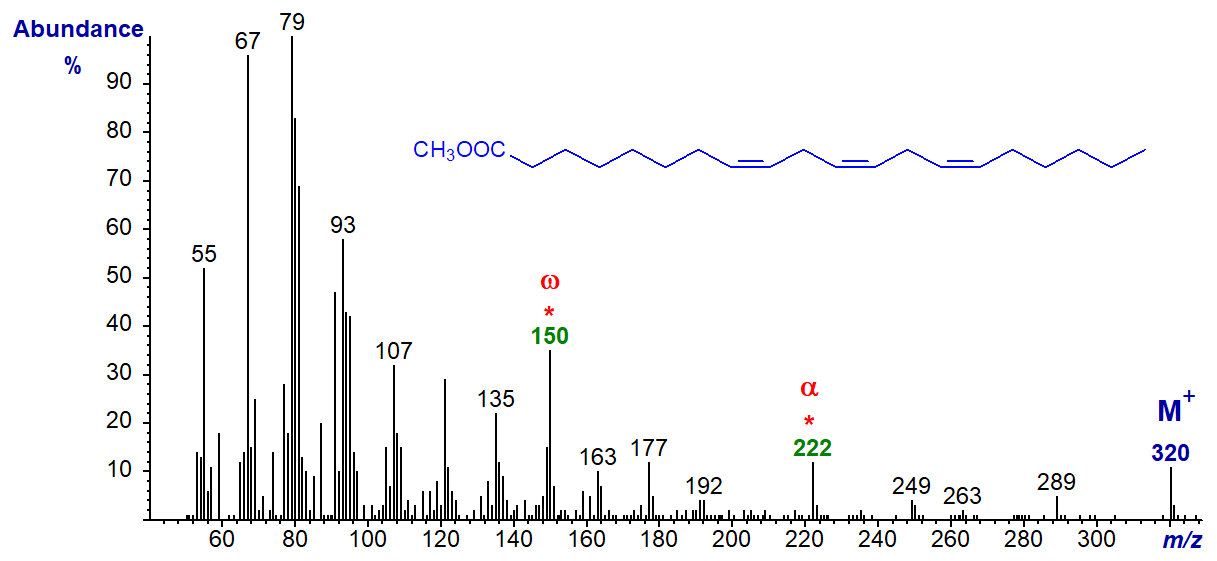
The mass spectrum of the methyl ester of 16:3(n-6) (or 4,7,10-16:3) is an exception in that it lacks the omega ion at m/z = 150, but has a distinctive if uncharacterized ion at m/z = 147 (spectrum not illustrated here but see the Archive pages); the alpha ion at m/z = 166 is present.
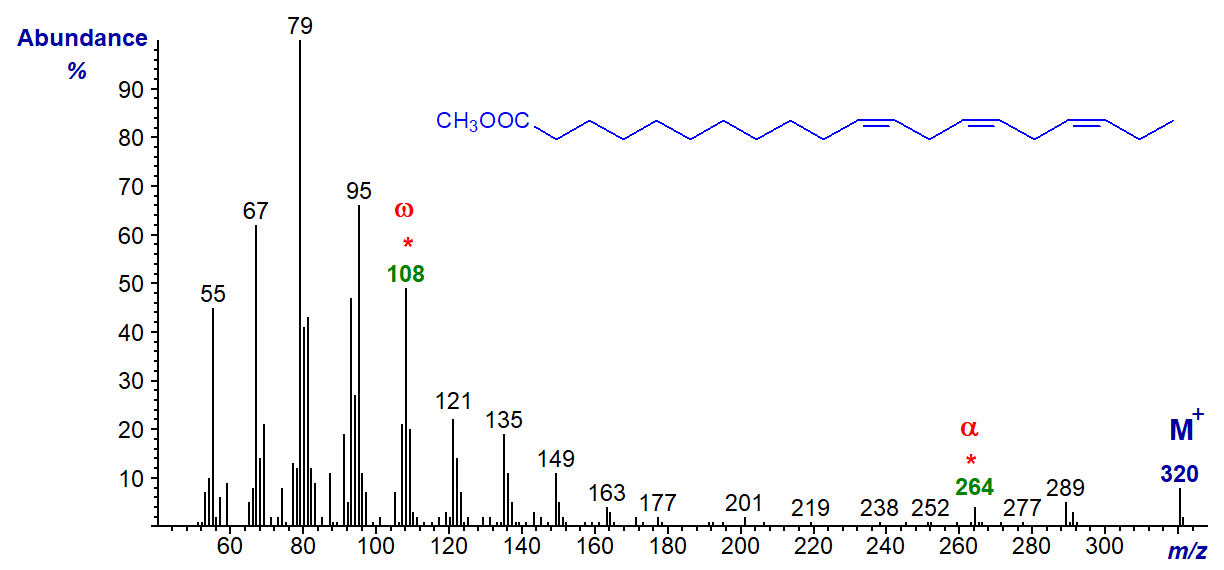
In the mass spectrum of methyl 11,14,17-eicosatrienoate (20:3(n-3)), the ion at m/z = 108 is distinctive, and the alpha ion for a Δ11,14 fatty acid at m/z = 264 is small but clear.
Polyunsaturated fatty acids of the n-9 family are less common in nature, but they do have biological relevance, especially in essential fatty acid deficiency in animals. The mass spectrum of the important isomer methyl 5,8,11-eicosatrienoate (Mead's acid or 20:3(n-9)), produced in this condition, is -
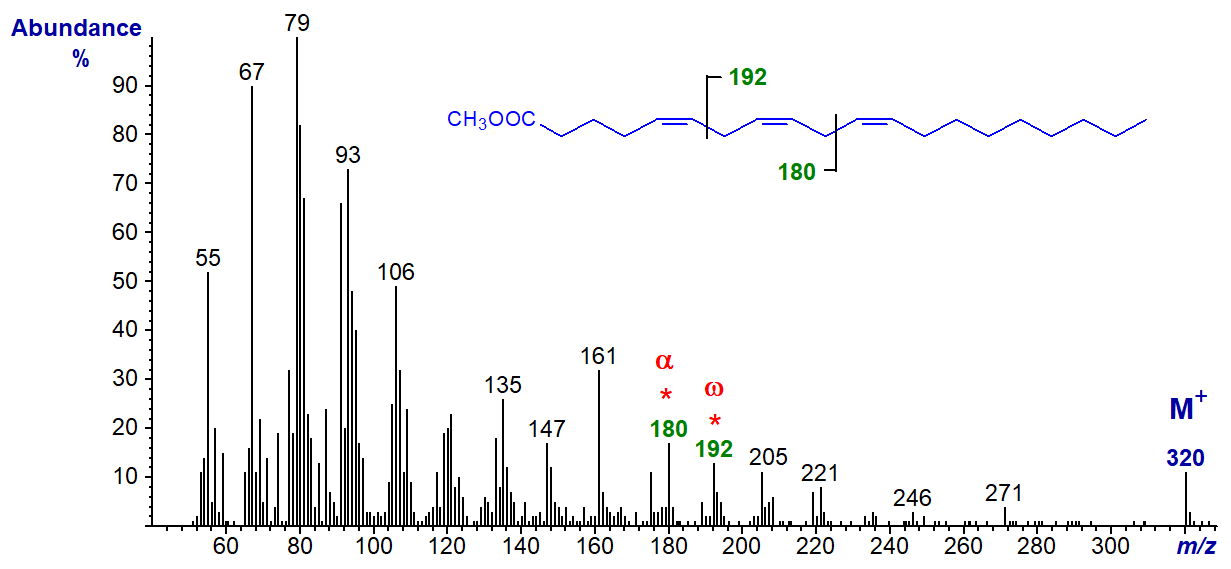
In this instance, the distinctive omega ion is that at m/z = 192, which is formed by an analogous type of cleavage to the key ions for the n-6 and n-3 families, while the alpha Δ5,8 ion is at m/z = 180. The origin of the ion at m/z = 161 is a puzzle.
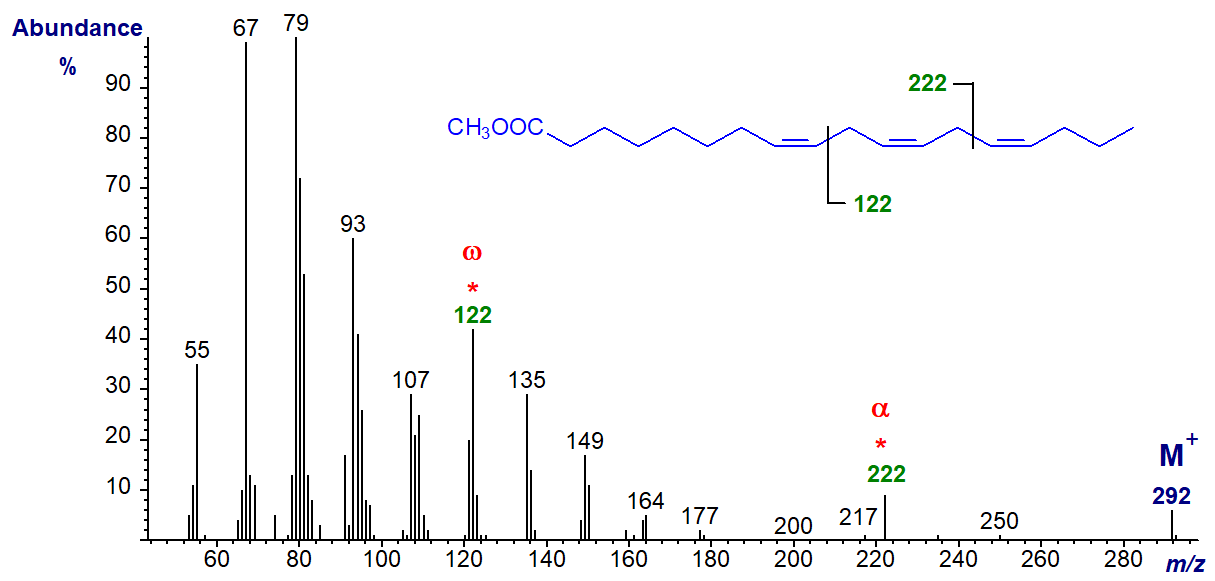
With the minor (n-4) family of fatty acids from fish oils, the diagnostic omega ion is at m/z = 122, as in the spectrum of methyl 8,11,14-octadecatrienoate, while the alpha Δ8,11 ion now is at m/z = 222 -
The spectrum of methyl 11,14,17-octadecatrienoate (18:3(n-1)) from a genetically modified organism is illustrated next to complete this section (Sayanova et al., 2006).
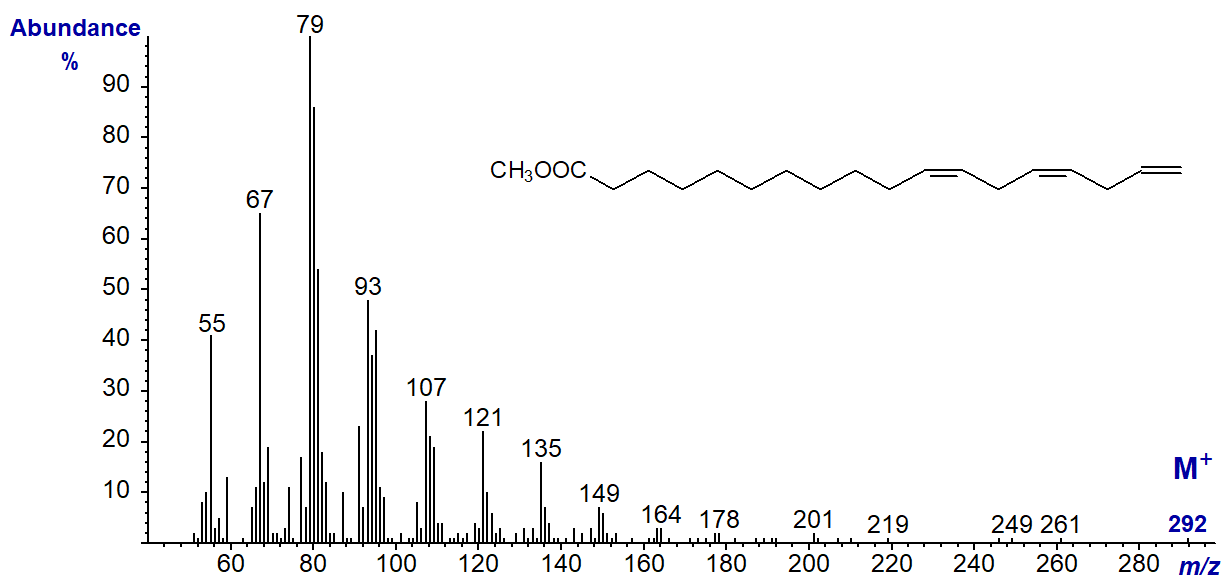
The omega ion is expected to be at m/z = 80, but this is present in the spectra of all methylene-interrupted polyunsaturated fatty acids so it is not suitable for characterization purposes in this instance; the alpha ion would be expected at m/z = 264 but is not present. This spectrum could be said to be distinct from those of the other isomers only in the sense that there appears to be nothing distinctive. Although trienes of the (n-1) family of fatty acids have yet to be found in nature, 18:4(n-1) is perhaps surprisingly a common minor constituent of fish oils.
We have spectra of the methyl esters of 7,10,13-hexadecatrienoate (16:3(n-3)), 16:3(n-1), 20:3(n-6), 20:3(n-3) and 22:3(n-6) in the Archive section but without interpretation.
Trienes with Di- and Polymethylene-Interrupted Double bonds
Although there may be some similarities, the rules developed for mass spectra of methylene-interrupted trienes do not apply when there is more than one methylene group between the double bonds, and insufficient spectra are available for definitive conclusions. Some reference spectra are available for comparison and study, and for example, 5,9,12-octadecatrienoate (5,9,12-18:3, pinolenic acid) is a common constituent of conifer lipids and its methyl ester has the spectrum shown next (Dobson and Christie, 2002) -
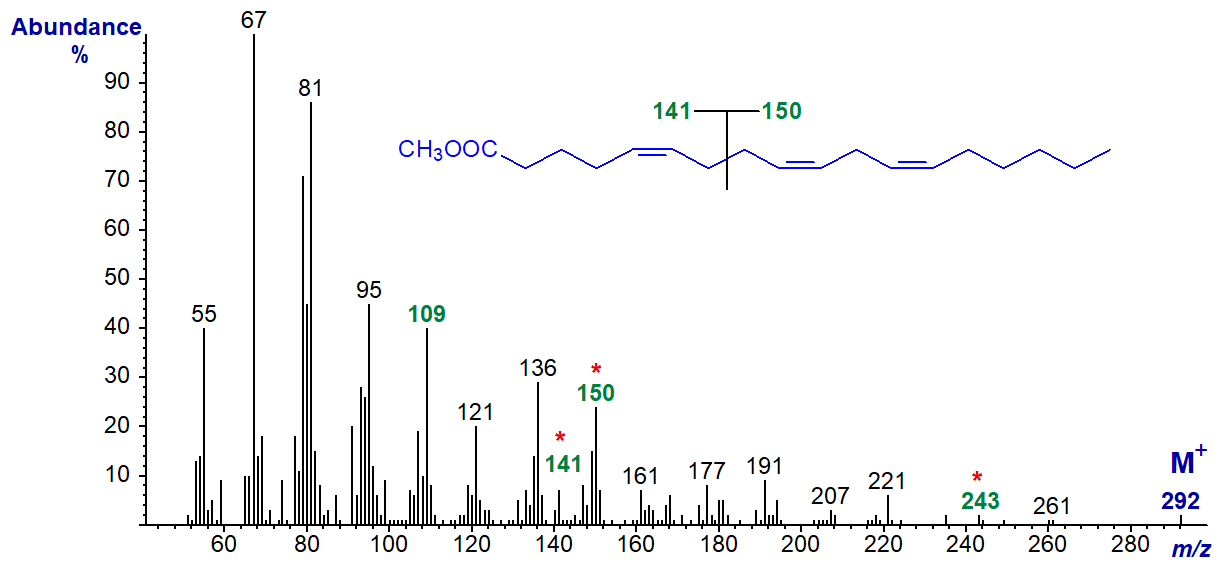
It has the ion at m/z = 150 for a (n-6) double bond system, and ions at m/z = 141, 109 and [M-49]+ (m/z = 243) characteristic of 5,9-dienes (see our web page on methyl esters of dienoic acids for a more detailed discussion). The key fragmentation is illustrated simplistically as occurring at the centre of the bis-methylene-interrupted double bond system, and this interpretation is supported by the spectrum of the ethyl ester (Archive), where the ion at m/z = 141 is shifted to 155, while that at m/z = 150 is unchanged.
We have mass spectra for 5,11,14-20:3, 7,11,14-20:3 (from conifers), 5,9,21-28:3, 5,9,22-29:3 and 5,9,23-30:3 (from sponges) and 5,13,17-20:3 (from a fish oil concentrate) - see our Archive page. Some of these fatty acids have the characteristic ions for a 5,9-double bond system, and the spectrum of one of them, methyl 5,9,23-triacontatrienoate (5,9,23-30:3), is illustrated.
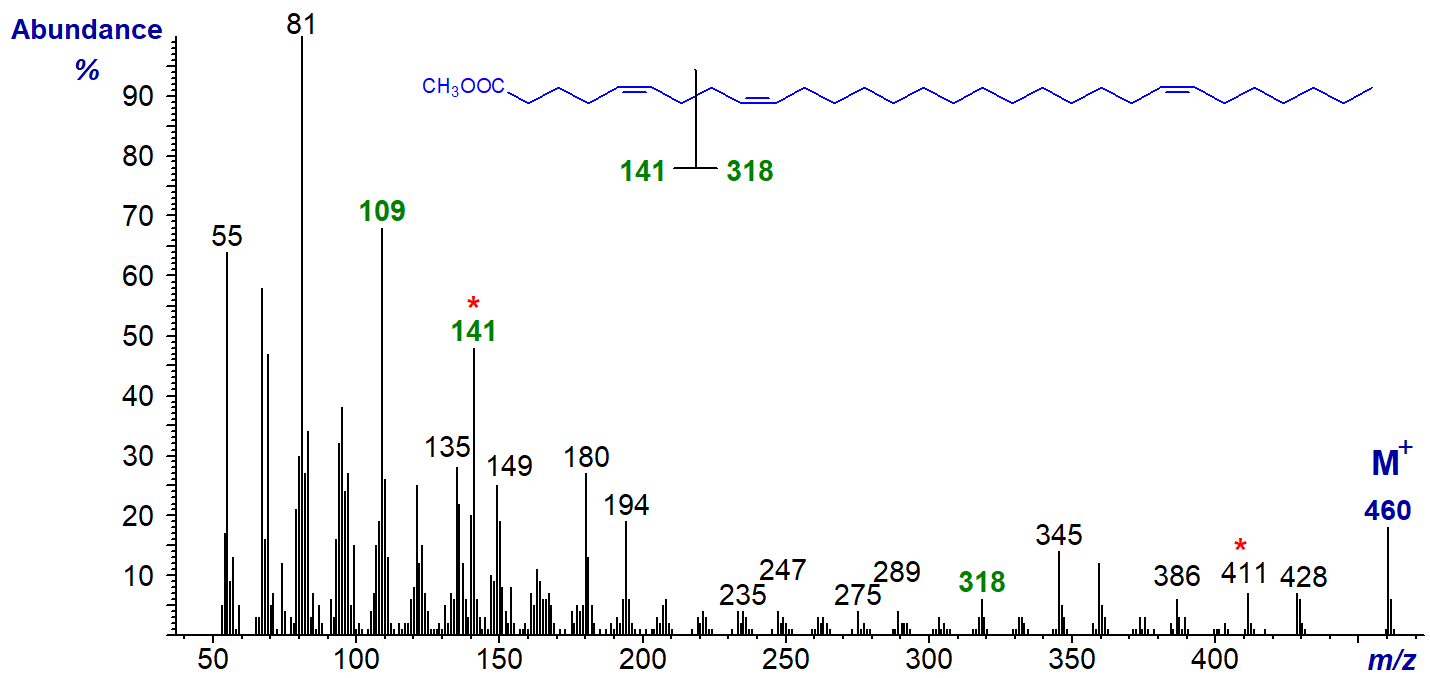
Thus, the key fragmentation ions are at m/z = 141 (and 109) and 318, while that for [M-49]+ (m/z = 411) is a useful aid to identification. Of course, it is unlikely that there are any diagnostic ions for the double bond in position 23.
The mass spectrum of methyl 7,11,14-eicosatrienoate (7,11,14-20:3) from the seed oil of Pinus contorta is -
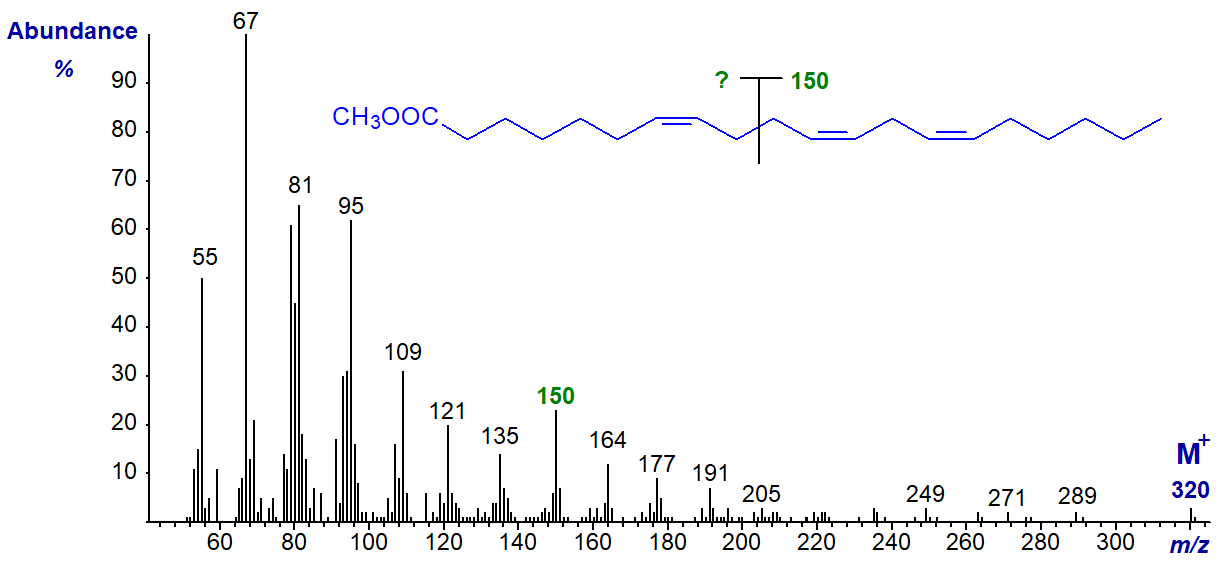
It could be argued that the ω-ion at m/z = 150 is present, but in contrast to the spectra of 5,9-isomers, there is nothing to suggest the presence of an α‑ion. As the spectrum is distinctly different from that of 8,11,14-20:3 illustrated earlier, it may serve as a fingerprint for comparison purposes.
3,9,12-Octadecatrienoic acid (usually with the first double bond of the trans-configuration, but here cis-) is occasionally found in seed oils from the Chrysanthemum and related families. The methyl ester has the spectrum (Dobson and Christie, 2002) -
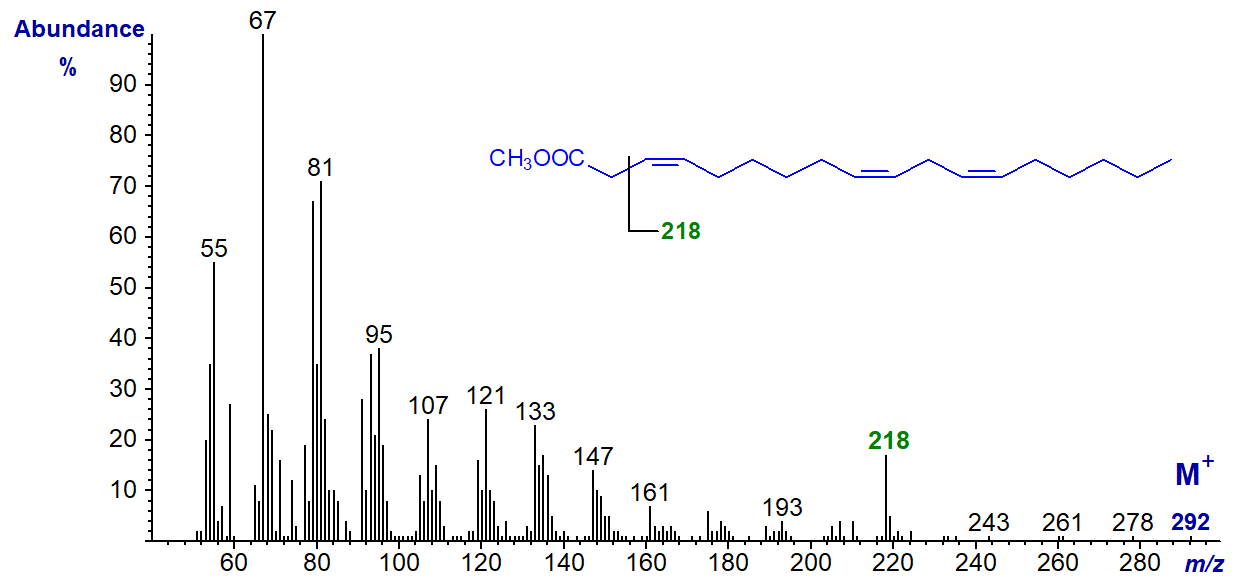
It does not resemble the spectra of other C18 trienes, and the ion at m/z = 218 is probably formed from a fragmentation in the position illustrated, i.e., after loss of the McLafferty ion (see also the spectrum of the analogous tetraene). The spectrum of the trans-3 isomer is identical to this as expected.
Trienes with Conjugated Double bonds
Trienoic fatty acids in which only two of the double bonds are in conjugation are rare in nature, and the best known is cis-9,trans-11,cis-15-octadecatrienoic acid. This is formed as an intermediate in the biohydrogenation of α-linolenic acid by microorganisms in the rumen and is a minor component of meat and dairy products from ruminant animals, such as sheep and cows. The mass spectrum of its methyl ester is -
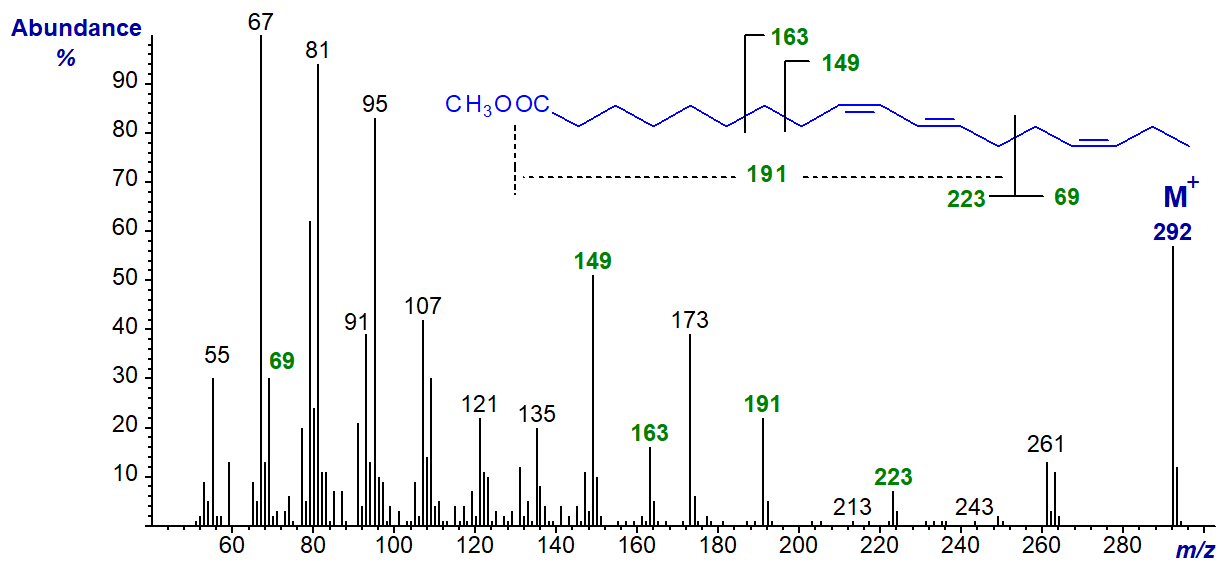
In this instance, the spectrum is quite informative, and the following interpretation seems probable. The ion at m/z = 223 represents a fragmentation at the centre of the bis-methylene-interrupted system containing the carboxyl group (the other expected ion at m/z = 69 is present but hidden among the low mass hydrocarbon ions). The ion at m/z = 191 represents loss of a methoxyl group from the ion at m/z = 223, while that at m/z = 173 represents a further fragmentation in which the elements of water are lost. The ions at m/z = 149 and 163 are formed from the hydrocarbon tail of the molecule involving cleavages beta and gamma, respectively, to the first double bond.
On the other hand, the spectrum of methyl 6-cis,9-cis,11-trans-octadecatrienoate, prepared by incubation of 9-cis,11-trans-octadecadienoic acid with the cyanobacterium Spirulina platensis, is less useful (author, unpublished).
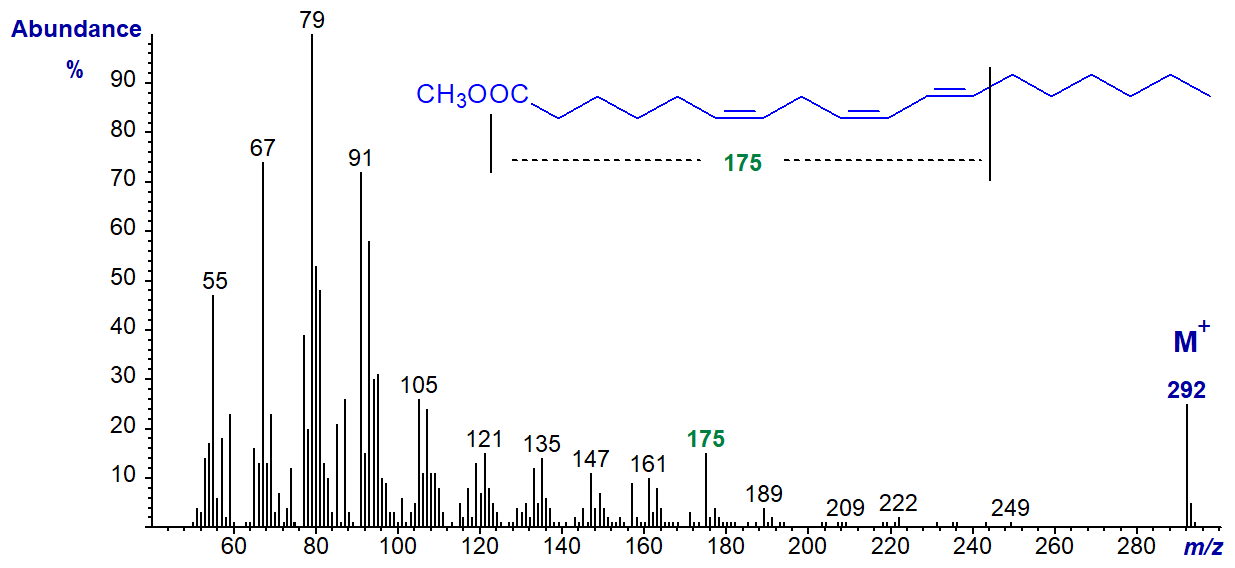
There is a distinctive ion at m/z = 175, and it is tempting to suggest that its origin is from a fragmentation at the bonds illustrated on the figure, although this should not be considered definitive.
Trienoic fatty acids with three double bonds in conjugation are important constituents of certain seed oils of commerce. The mass spectrum of the methyl ester of the conjugated triene, α‑eleostearate, or methyl 9-cis,11-trans,13-trans-octadecatrienoate is -
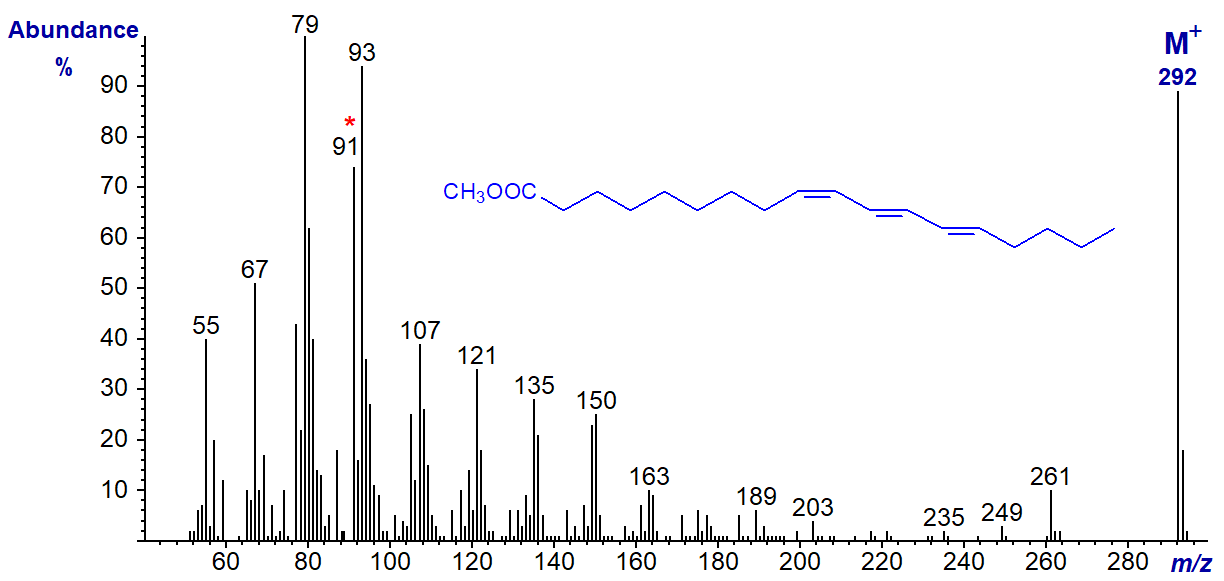
There are two main distinctive features, a particularly high molecular ion and a tropylium rearrangement ion at m/z = 91 (discussed in our web document on mass spectrometry of tetraenes and present in the previous two spectra). Both ions are characteristic of highly conjugated double bond systems, but there are no ions indicative of double bond positions. Other conjugated trienes differing in both the positions and geometry of the double bonds have mass spectra that closely resemble this.
Mass spectra of the MTAD adducts of the methyl esters of the conjugated triene, punicic acid, are described in another document (see the section on Mass spectra of methyl esters of fatty acids - further derivatization).
We have spectra of many more methyl esters of trienoic fatty acids of various chain lengths on file, and they can be accessed (but without interpretation) from our Archive page.
References
 Brauner, A.,
Budzikiewicz, H. and Boland, W. Studies in chemical ionization mass spectrometry.
5. Localization of homoconjugated triene and tetraene units in aliphatic compounds. Org. Mass Spectrom., 17,
161-164 (1982); DOI.
Brauner, A.,
Budzikiewicz, H. and Boland, W. Studies in chemical ionization mass spectrometry.
5. Localization of homoconjugated triene and tetraene units in aliphatic compounds. Org. Mass Spectrom., 17,
161-164 (1982); DOI.- Dobson, G. and Christie, W.W. Mass spectrometry of fatty acid derivatives. Eur. J. Lipid Sci. Technol., 104, 36-43 (2002); DOI.
- Fellenberg, A.J., Johnson, D.W., Poulos, A. and Sharp, P. Simple mass spectrometric differentiation of the n-3, n-6 and n-9 series of methylene interrupted polyenoic acids. Biomed. Environ. Mass Spectrom., 14, 127-130 (1987); DOI.
- Hallgren, B., Ryhage, R. and Stenhagen, E. The mass spectra of methyl oleate, methyl linoleate and methyl linolenate. Acta Chem. Scand., 13, 845-847 (1959); DOI.
- Holman, R.T. and Rahm, J.J. Analysis and characterization of polyunsaturated fatty acids. Prog. Chem. Fats Other Lipids, 9, 15-90 (1971); DOI.
- Sayanova, O., Haslam, R., Guschina, I., Lloyd, D., Christie, W.W., Harwood, J.L. and Napier, J.A. A bifunctional Δ12,Δ15-desaturase from Acanthamoeba castellanii directs the synthesis of highly unusual n-1 series unsaturated fatty acids. J. Biol. Chem., 281, 36533-36541 (2006); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
