Mass Spectrometry of Methyl Esters
Dienoic Fatty Acids
As cautioned in the Introduction to these documents, the mass spectra of methyl esters obtained with electron-impact ionization afford limited information only concerning the structures of unsaturated fatty acids. However, the molecular weight is usually obtainable, and this is a significant piece of information, and the pattern of ions in spectra can be often useful as general 'fingerprints'. If chromatographic retention data are added to this, it is often possible to be 90% certain of the identity of a fatty acid. In addition, there are a few key ions that help to identify specific diunsaturated fatty acids, though these must be applied with caution. A few of the spectra illustrated below may have been published elsewhere, but it would be an onerous task to establish priority, and most are unique to this web site.
Methylene-Interrupted Dienes
As with monoenes, mass spectra of methyl esters of the common methylene-interrupted dienoic fatty acids do not allow ready interpretation in terms of double bond positions, althoughsmall differences in spectra of other isomers are sometimes useful as fingerprints, which can be related to some structural aspect. The mass spectrum of methyl linoleate (9,12-18:2 or 18:2(n-6)) is illustrated below (Hallgren et al., 1959) -
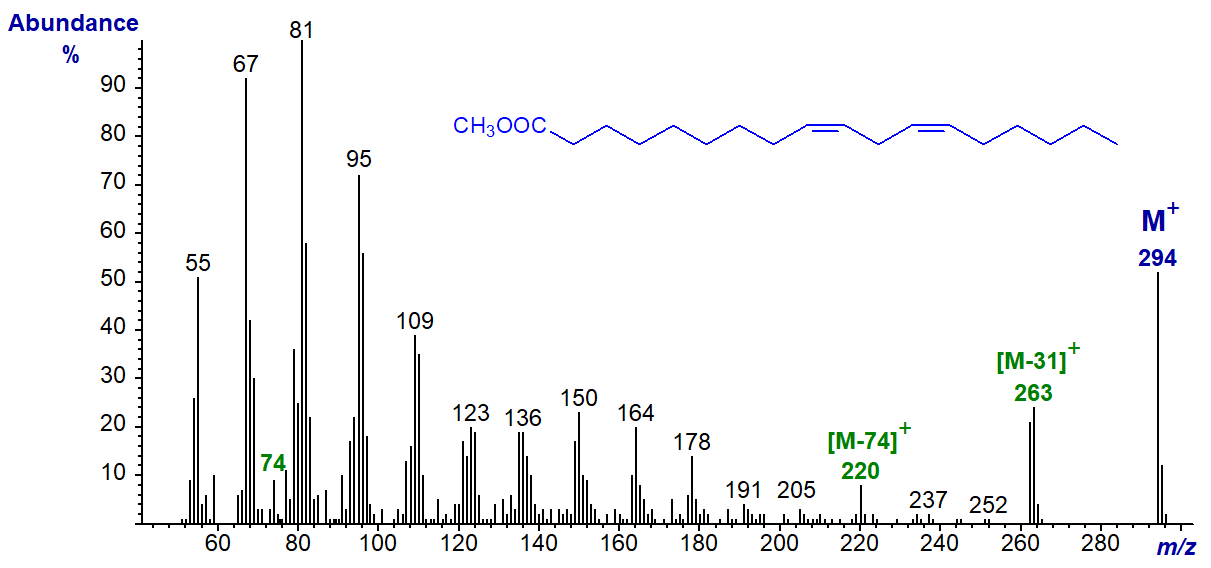
It has an abundant molecular ion (m/z = 294) and a prominent ion for loss of the McLafferty ion (m/z = 220), although the McLafferty ion per se (m/z = 74) is small. The ion representing [M‑31]+ is higher than that for [M‑32]+ (although this may not be true for all isomers). Hydrocarbon ions of general formula [CnH2n‑3]+ dominate in the lower mass range (m/z = 67, 81, 95, 109, 123, etc). As with the monoenes, there is little or no evidence for any ions that might serve to locate the double bonds, and those ions found to be useful diagnostic aids for methylene-interrupted polyenoic fatty acids, i.e., those with 3 to 6 double bonds (see the web pages on methyl esters of trienes), do not apply for dienes.
Mass spectra of the methyl esters of all the C18 methylene-interrupted dienes have been described, and those with the double bonds close to the carboxyl group, i.e., 2,5-18:2 to 5,8-18:2, differed significantly from the rest (Christie and Holman, 1967), but it would be difficult to describe these differences in mechanistic terms in relation to particular double bond positions, other than perhaps the first; they are illustrated in our Archive pages. As an example, the mass spectrum of methyl 5,8‑octadecadienoate (sebaleic acid), which occurs naturally in sebum -
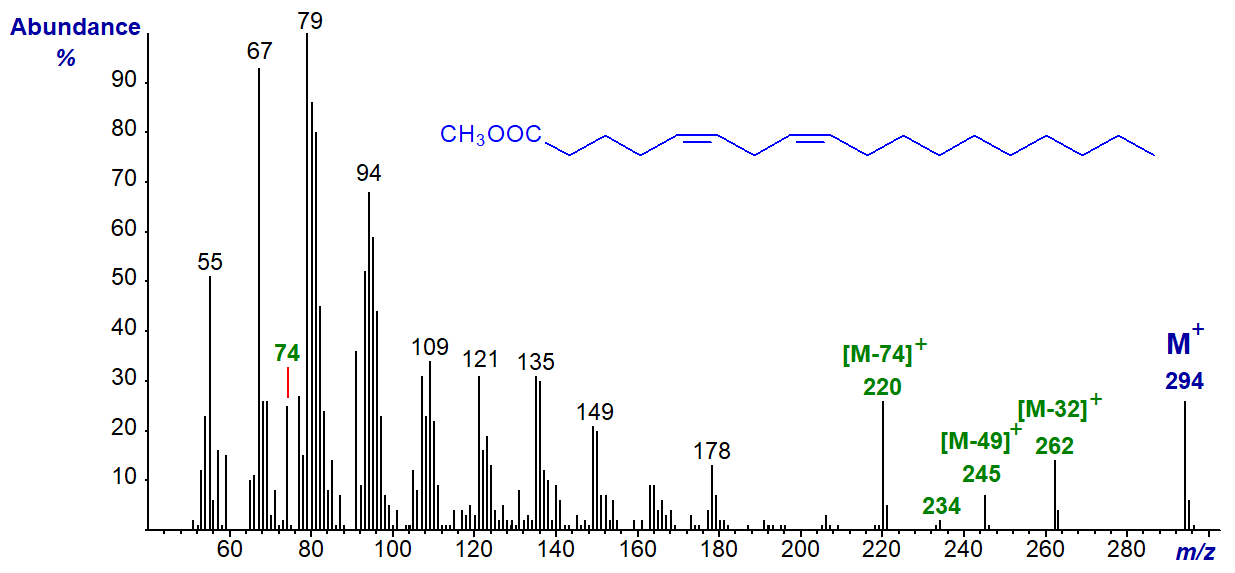
The McLafferty ion at m/z = 74 and that for the loss of the McLafferty ion at m/z = 220 are especially abundant in this and the spectra of the 2,5- to 4,7-isomers. Similarly, ions at m/z = 245 ([M‑49]+) and 234 ([M‑60]+) are found at significant levels only when the double bonds are relatively close to the carboxyl group. While by no means definitive, the pattern or fingerprint of the latter ions and of ions in the low mass region is consistent in homologues such as 5,8-17:2 and 5,8-19:2, and it should be sufficiently different from that of the 9,12-isomer to raise questions with someone seeing it as an unknown.
When the double bond is further from the carboxyl group, the differences between the spectra of isomers are trivial. Thus, the spectrum of methyl 11,14‑octadecadienoate is -
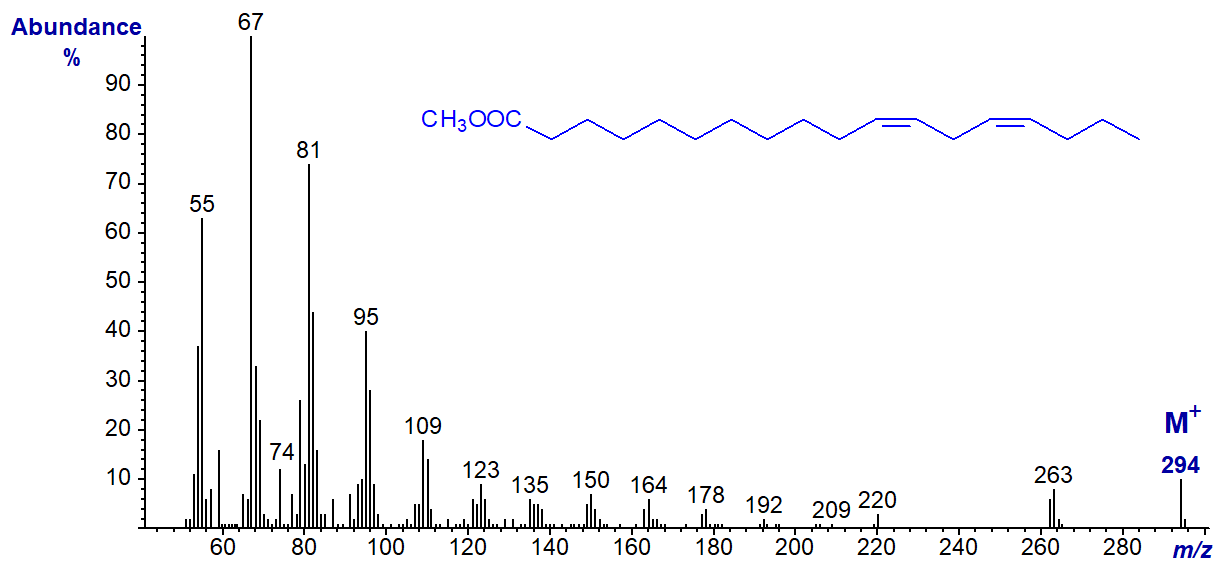
This spectrum resembles that of methyl linoleate above except that the ions in the higher mass range are of relatively lower intensity. Small differences are sometimes due to instrumental factors such as when the source was last cleaned.
The mass spectrum of methyl 7,10-hexadecadienoate (or 16:2(n-6)) is illustrated next for comparison.
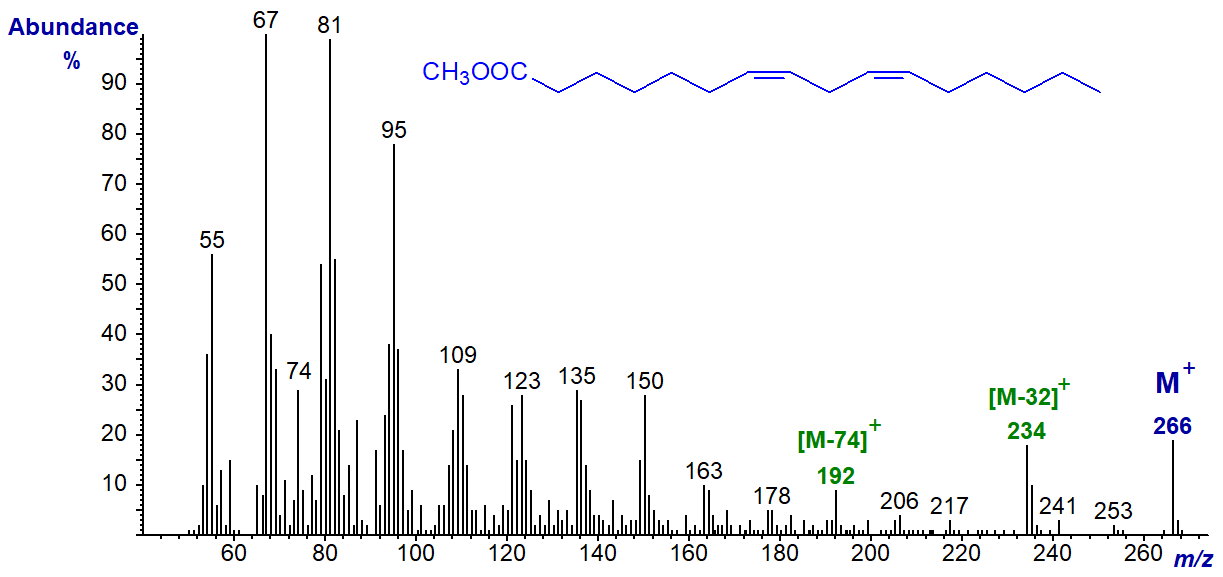
This spectrum closely resembles the previous one except that the key ions of higher mass are at 28 amu less, although the McLafferty ion is still relatively substantial (cf., the 5,8-isomer above).
One natural further diene of this type is of some biological interest, i.e., methyl 11,14-eicosadienoate (20:2(n-6)), which has the next spectrum. Again, this has no diagnostic features to aid double bond location.
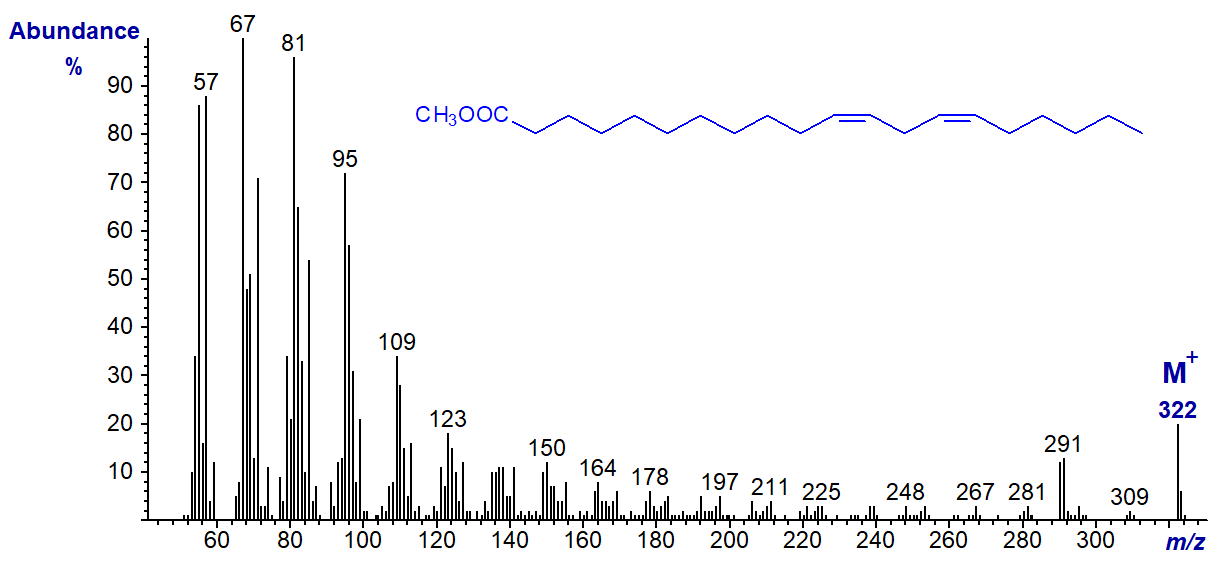
Bis-Methylene-Interrupted Dienes
Fatty acids with 5,9-double bond systems are common in sponges and conifers. The mass spectrum of methyl 5,9-octadecadienoate, i.e., with two methylene groups between the double bonds, is similar to that of the previous dienes in many ways, although it does have distinctive ions at m/z = 109, 141 and 245 ([M‑49]+), which appear to serve as guides or fingerprints for identification. That at m/z = 141 is presumably formed from the carboxyl end of the molecule by cleavage between carbons 7 and 8, i.e., at the centre of the double bond system. It is present in all homologous 5,9-dienoates, as is an ion at m/z = 109, which is equivalent to that at m/z = 141 less the elements of methanol (minus 32 amu). A loss of 49 amu from the molecular ion is presumably due first to the loss of both the methoxyl group and the elements of water from the molecular ion and occurs in the spectra of all dienes when the first double bond is close to the carboxyl group, as with the 5,8-isomer above. The base ion is usually at m/z = 81 rather than 67. While the spectrum below has not been published elsewhere to my knowledge, some aspects of the distinctive fragmentations have been discussed in connection with the spectrum of 5,9,12-18:3 (Dobson and Christie, 2002).
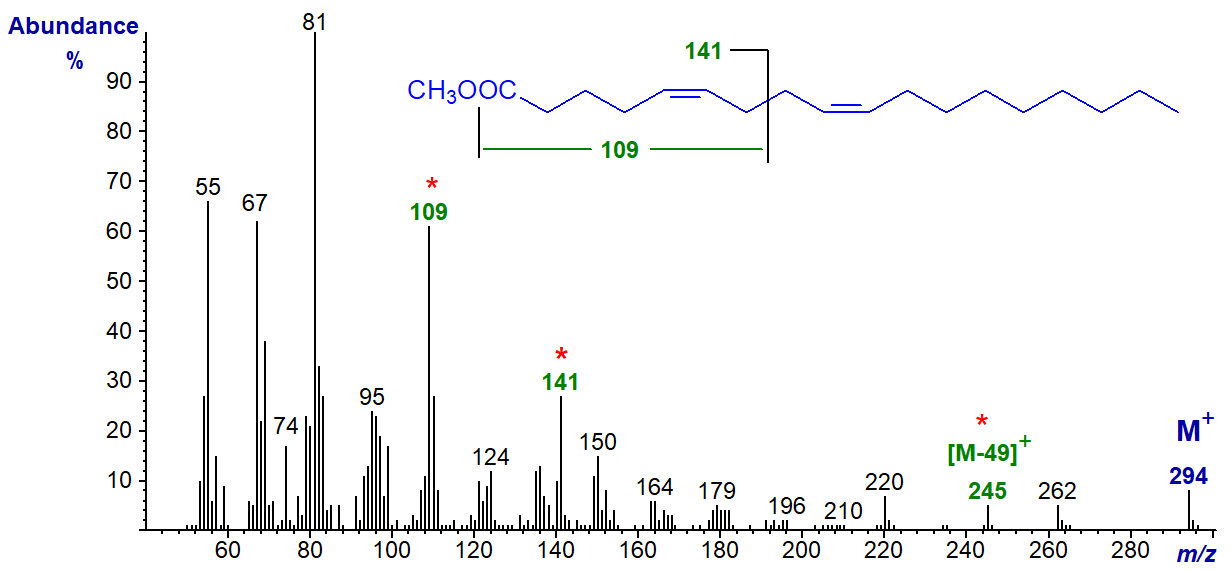
These interpretations are supported by the spectrum of the corresponding ethyl ester, in which the ions at m/z = 109 and 245 remain (the latter now at [M‑63]+), while that at m/z = 141 is shifted upwards by 14 amu to m/z = 155.
Analogous ions are present in the mass spectra of the methyl esters of 5,9-16:2, 5,9-17:2, 5,9-22:2, 5,9-23:2, 5,9-24:2, 5,9-25:2, 5,9-26:2, 5,9-27:2 and 5,9-28:2 and in related fatty acids with methyl branches; with the latter the ions for fragmentations at the branch points are not easily distinguished (all published here for the first time and obtained from analyses of sponge fatty acids - see the Archive page). The mass spectrum of the very-long-chain methyl 5,9-octacosadienoate is illustrated -
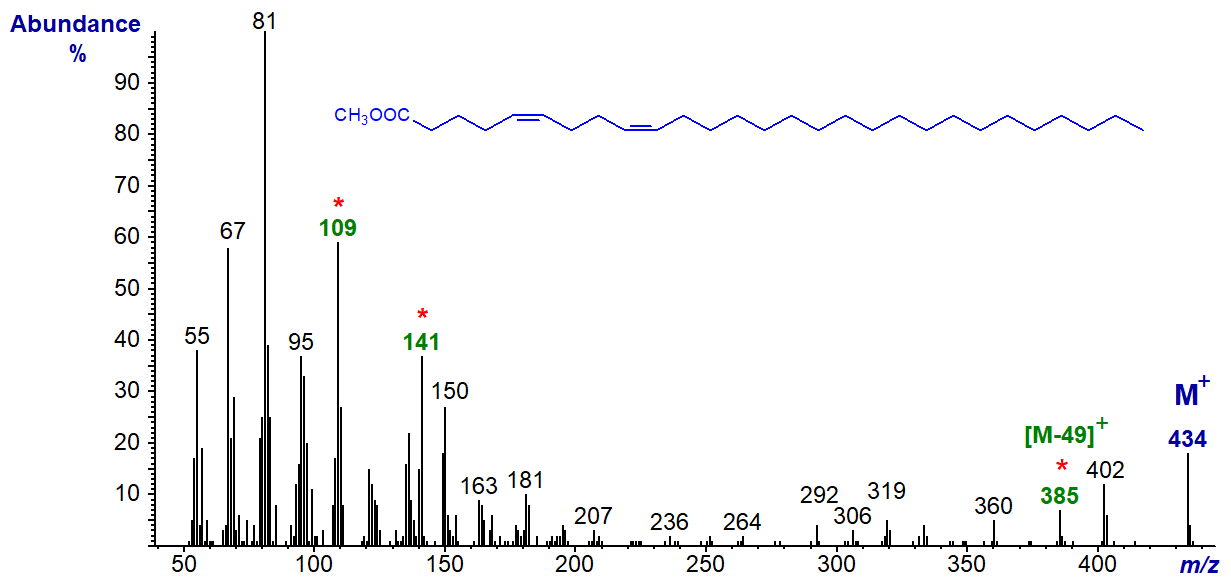
As noted earlier, the ions at m/z = 141 and 109 and that for [M-49]+ (m/z = 385) are useful diagnostically. It might be expected that there would be a companion ion to the former from the terminal part of the molecule, and it could be argued that this is represented by the small ion at m/z = 292. While comparable ions appear to be present in the spectra of related isomers, they are sometimes too indistinct to be certain of their origin. On the other hand, equivalent ions that are presumably formed in the same way are seen in the spectra of comparable isopropyl esters.
The mass spectrum of methyl 6,10-octadecadienoate (prepared by total synthesis in the laboratory of Professor M.S.F. Lie Ken Jie in Hong Kong) is -
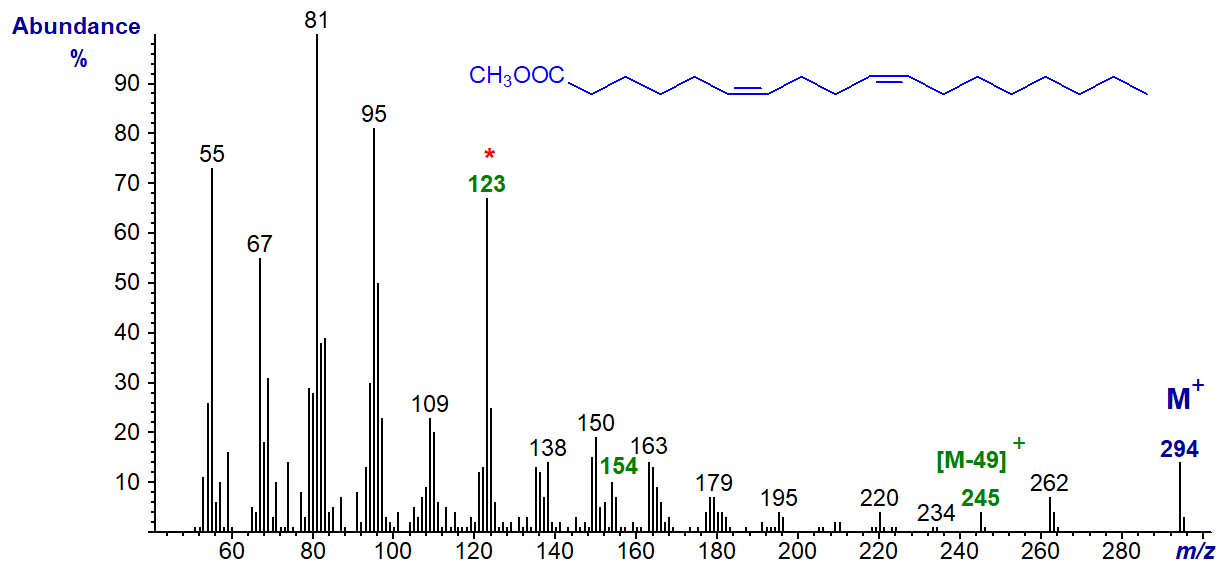
By analogy with the spectrum of the 5,9-analogue, we might expect a characteristic ion at m/z = 155. This does not stand out, but the ion at 32 amu less (m/z = 123) is presumably formed from it by the loss of the elements of methanol. In this instance too, an ion representing [M-49]+ (m/z = 245) is present.
Polymethylene-Interrupted Dienes
The mass spectrum of methyl trans-3,cis-9-octadecadienoate, a minor component in some seed oils of the Compositae family, is -
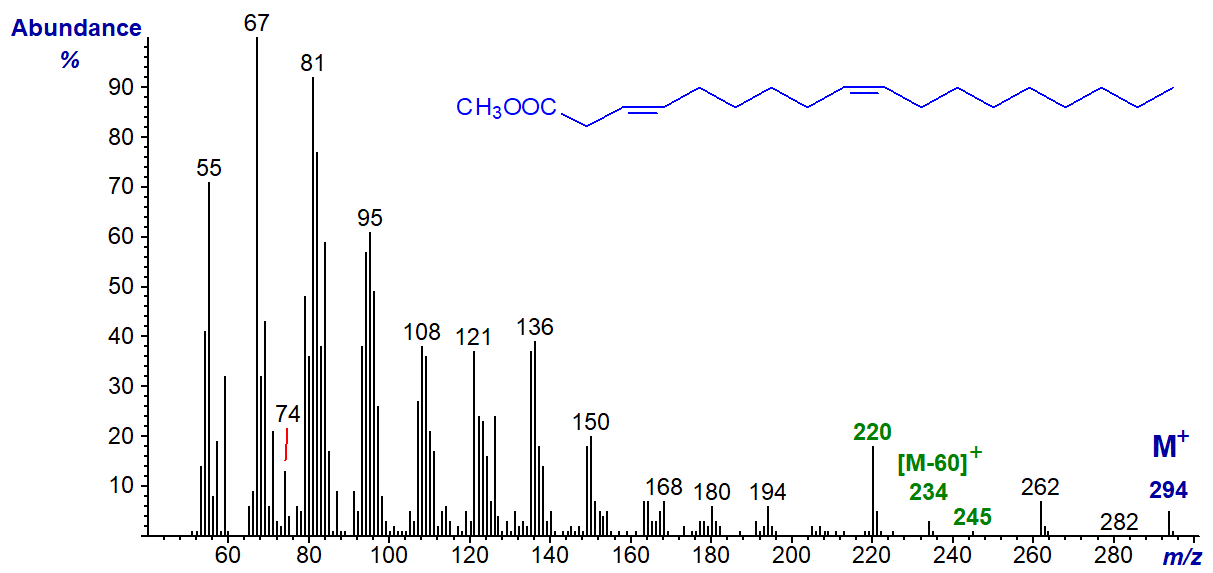
The differences from the spectrum of methyl linoleate above are trivial, mainly in the relative intensities of certain ions. For example, the molecular ion is smaller and the [M‑74]+ ion (m/z = 220) is larger, but there is nothing that suggests the location or configuration of any of the double bonds. There are ions for [M‑60]+ and [M‑49]+ at m/z = 234 and 245, respectively. If required, it would be possible to determine the positions of the double bonds via the dimethyl disulfide derivatives (not possible with methylene-interrupted dienes), as an alternative to 3‑pyridylcarbinol and other nitrogen-containing derivatives.
The mass spectrum of methyl 6,11-octadecadienoate (prepared by total synthesis in the laboratory of Professor M.S.F. Lie Ken Jie in Hong Kong) is -
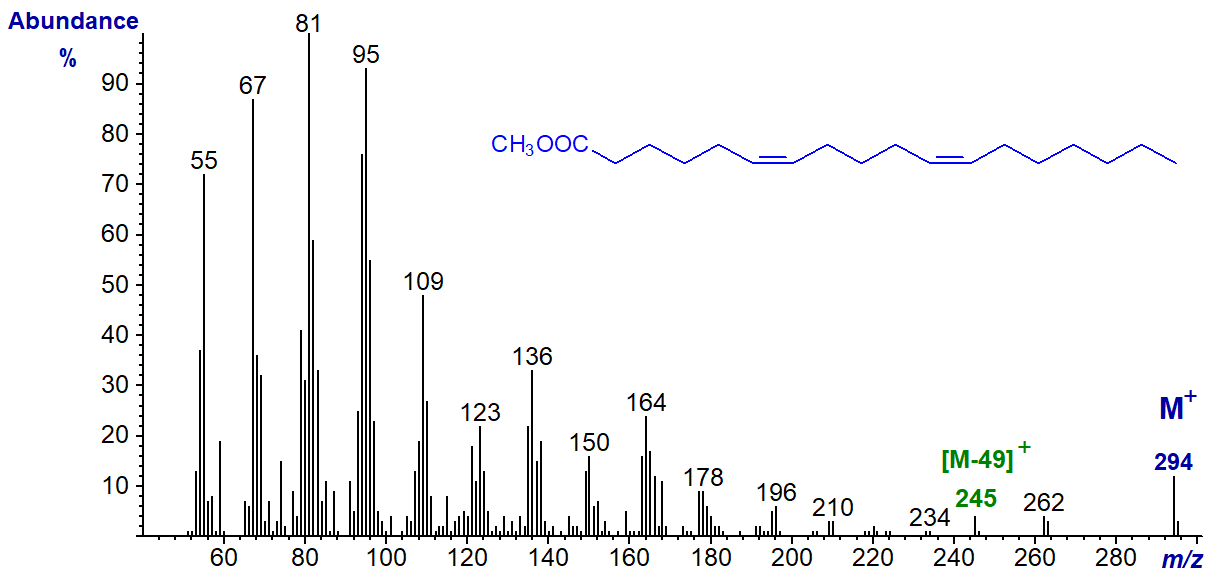
Again, it seems best to treat this simply as a fingerprint spectrum, as no interpretation in terms of double bond position seems possible. The spectrum resembles that of linoleate, although an ion representing [M‑49]+ is again apparent. This is not a consistent feature when three or more methylene groups separate the double bonds.
The spectrum of methyl 5,13-docosadienoate (from meadowfoam oil) is likewise undistinguished -
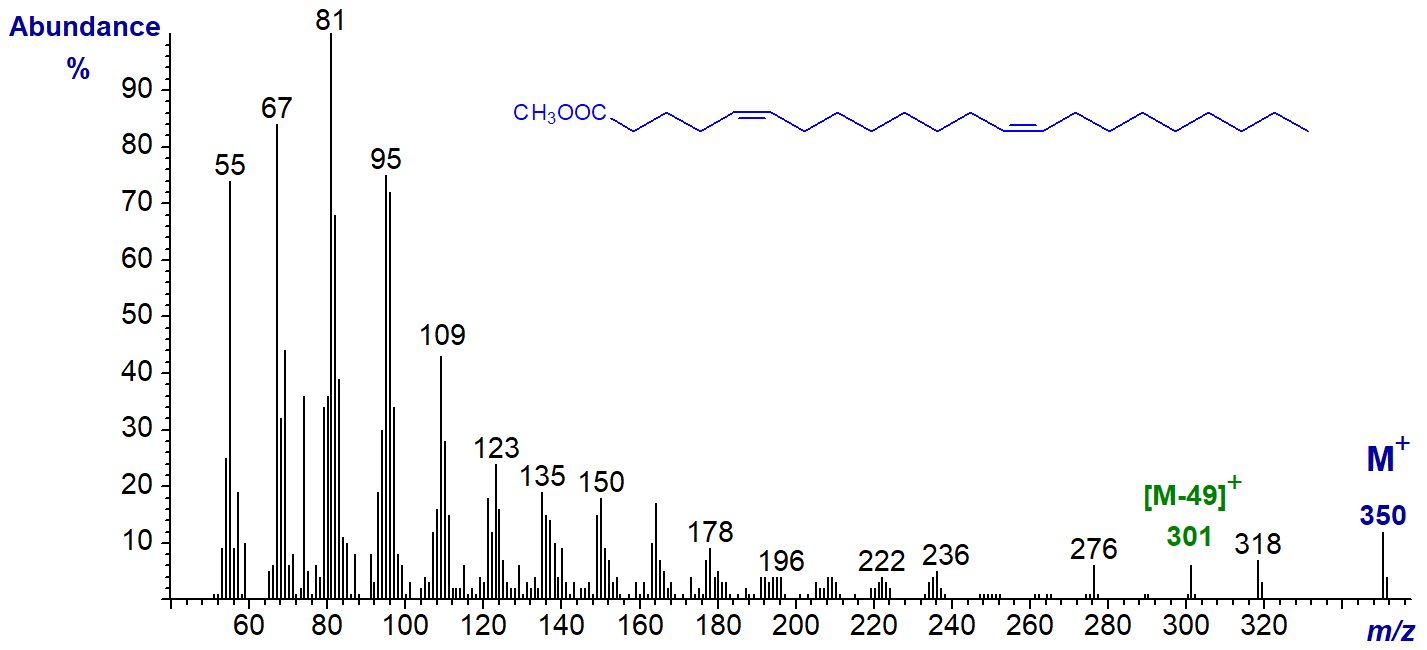
We have spectra of methyl esters of 5,12- and 7,12-18:2 (synthetic) on file in the Archive section; they lack an [M‑49]+ ion.
Conjugated Dienes
The mass spectrum of the methyl ester of a fatty acid with a conjugated diene system is illustrated, i.e., methyl 9-cis,11-trans-octadecadienoate (first published by Iversen et al., 1984). This is a natural component of all ruminant fats as a product of the biohydrogenation process in the rumen, and it is a major component of commercial ‘conjugated linoleic acid’ (CLA) preparations.
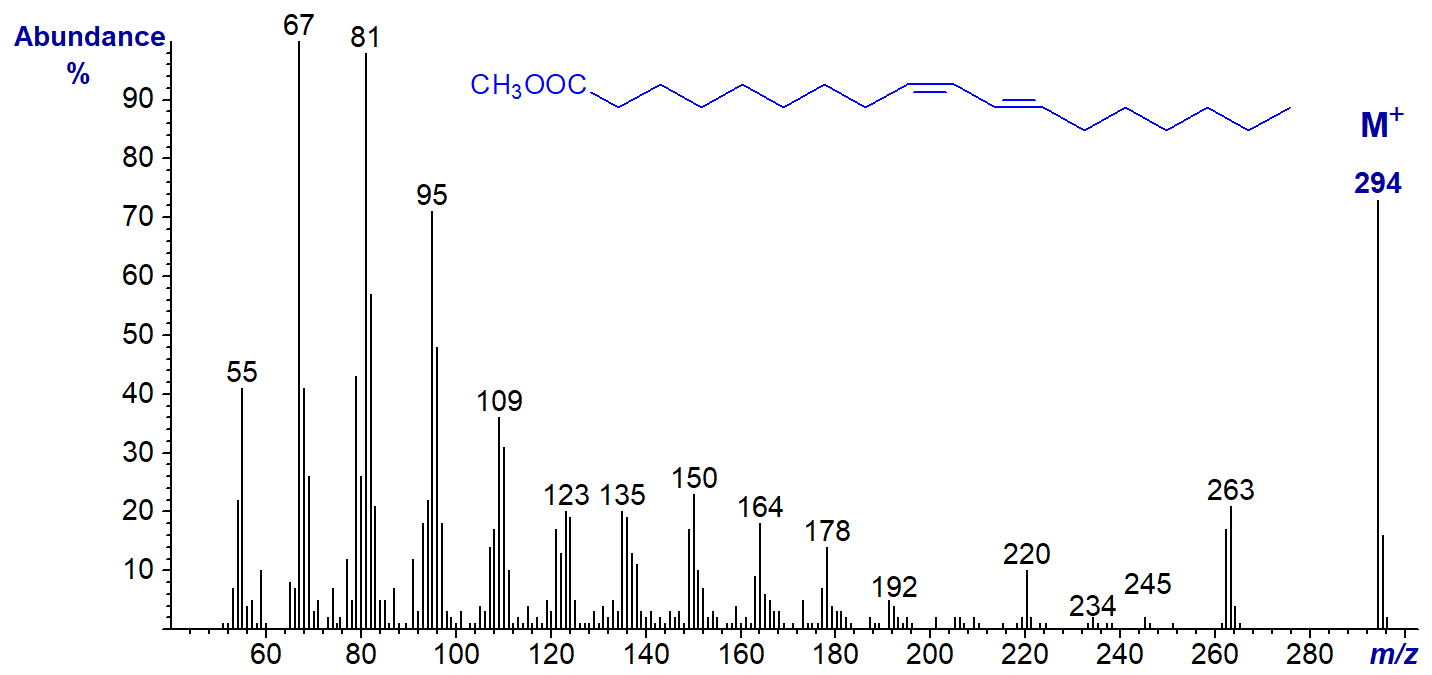
Here, the only distinguishing feature from the spectrum of linoleate seems to be a more abundant molecular ion. No useful information on the positions of the double bonds can be gleaned from the spectrum, and that of methyl 10-trans,12-cis-18:2 is identical to this (not illustrated). Double bond positions can be determined after a further derivatization step, i.e., the preparation of 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) adducts (see the section on Mass spectra of methyl esters of fatty acids - further derivatization).
We have spectra of many more methyl esters of dienoic fatty acids on file, and they can be accessed (but without interpretation) from our Archive page.
Chemical ionization with acetonitrile as the reagent gas can enable both double bond position and geometry to be determined as reviewed by Brenna (2006). I have no personal experience of this methodology so cannot comment further.
References
- Brenna, J.T. Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent adduct chemical ionization. In: Lipid Analysis and Lipidomics: New Techniques and Applications. pp. 157-172 (edited by: M.M. Mossoba, J.K.G. Kramer, J.T. Brenna, and R.E. McDonald, AOCS Press, Champaign, USA) (2006).
- Christie, W.W. and Holman, R.T. Synthesis and characterization of the complete series of methylene-interrupted cis,cis-octadecadienoic acids. Chem. Phys. Lipids, 1, 407-423 (1967); DOI.
- Dobson, G. and Christie, W.W. Mass spectrometry of fatty acid derivatives. Eur. J. Lipid Sci. Technol., 104, 36-43 (2002); DOI.
- Hallgren, B., Ryhage, R. and Stenhagen, E. The mass spectra of methyl oleate, methyl linoleate and methyl linolenate. Acta Chem. Scand., 13, 845-847 (1959); DOI.
- Iversen, S.A., Cawood, P., Madigan, M.J., Lawson, A.M. and Dormandy, T.L. Identification of a diene conjugated component of human lipid as octadeca-9,11-dienoic acid. FEBS Letts, 171, 320-324 (1984); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
