Mass Spectrometry of DMOX Derivatives
Dienoic Fatty Acids. Part 1. Methylene-Interrupted Dienes
The electron-impact mass spectra of 4,4-dimethyloxazoline (DMOX) derivatives of dienoic fatty acids, like those of monoenes, tend to be distinctive and permit location of the double bonds, especially when they are present in central positions. The same rules that were applied to the location of double bonds in monoenes are applicable here. Few spectra of this type have been published formally in the scientific literature. Here in Part 1, the spectra of methylene-interrupted dienes of various chain-lengths are discussed, while Part 2 describes the mass spectra of the DMOX derivatives of the other types of dienoic acids that were available to us.
Methylene-Interrupted Octadecadienoates
With all unsaturated fatty acids, interpretation of spectra when double bonds are near either end of the molecule can be more difficult, but the task should be greatly simplified by publication here of details of the mass spectra of the DMOX derivatives of a comprehensive series of positional isomers of methylene-interrupted octadecadienoic acids (4,7- to 14,17-18:2), which were prepared some years ago by total synthesis (Christie and Holman, 1967). Fortunately, even when all double bonds cannot be located with certainty from first principles, each isomer has a distinctive fingerprint that can be used for comparison purposes with published spectra.
The spectrum of the DMOX derivative of the ubiquitous 9,12-octadecadienoate (linoleate) is illustrated first (Zhang et al., 1988).
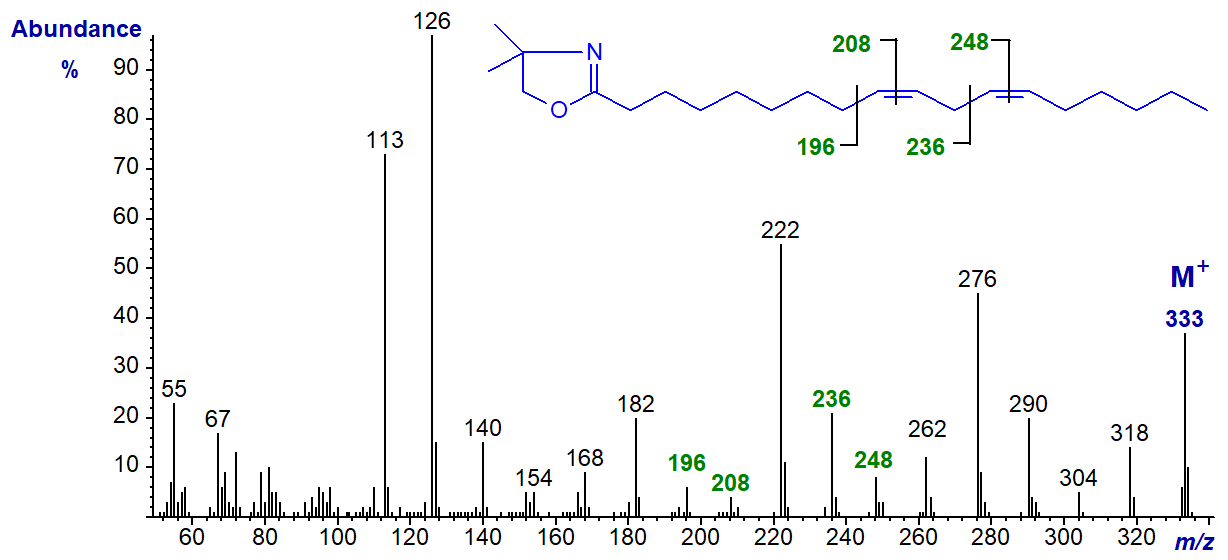
Double bonds are located by finding the gaps of 12 amu as described for monoenoic fatty acids (Mass spectra of DMOX derivatives. Monoenoic fatty acids). Thus, in this instance they are located between m/z = 196 and 208, and 236 and 248, for double bonds in positions 9 and 12, respectively. The prominent ions at m/z = 222 and 276 are formed by rearrangements that give stable conjugated double bond systems, and these and the analogous ions for other isomers can be useful diagnostic indicators when fatty acids are not fully resolved by gas chromatography or when the background noise level is high. The abundant ions at m/z = 113 and 126 are common to most DMOX derivatives, as described on the web page dealing with saturated derivatives.
Mass spectra of the DMOX derivatives of the other isomers follow with brief comments. When the first double bond is close to the carboxyl group, the 'fingerprint' spectrum for the appropriate monoene derivative is an invaluable guide to identification.
The DMOX derivative of 4,7-octadecadienoate (4,7-18:2) -
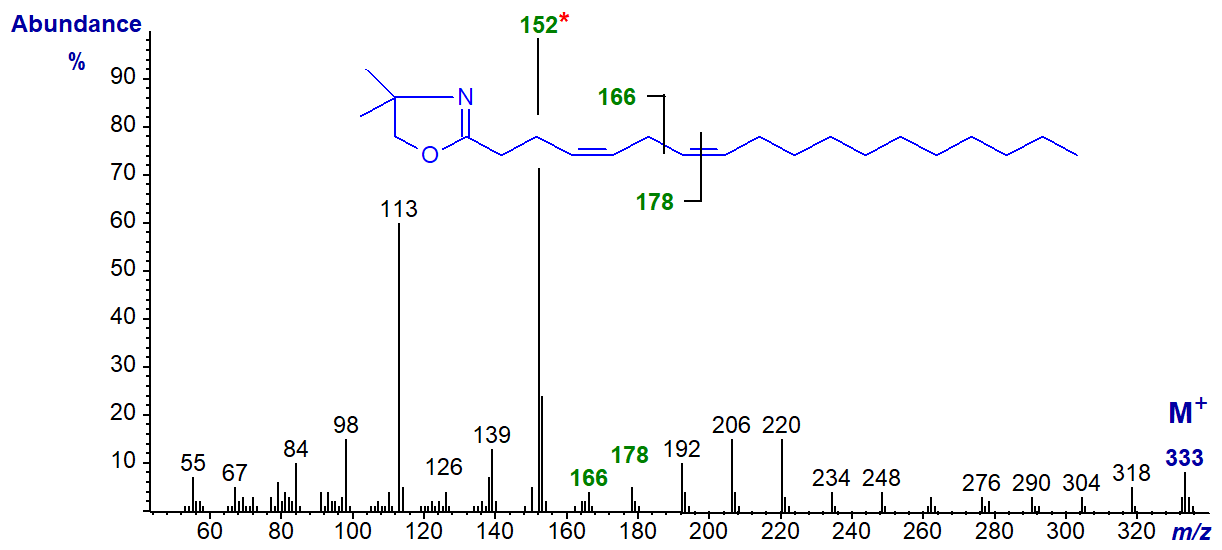
In this instance, the spectrum is dominated by an ion at m/z = 152, which may be diagnostic for the double bond in position 4. The double bond in position 7 is clearly located by the gap of 12 amu between m/z = 166 and 178. Thereafter, the ions are 14 amu apart for each successive methylene group.
The DMOX derivative of 5,8-octadecadienoate (5,8-18:2 or ‘sebaleic’ acid), a unique component of human skin wax -
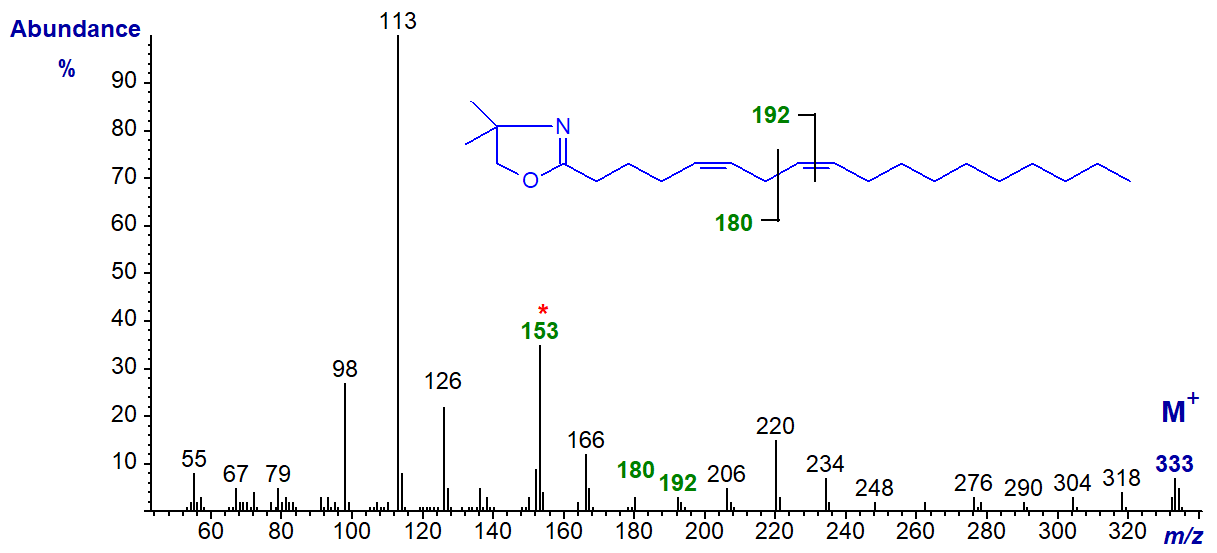
The double bond in position 8 is located by the gap of 12 amu between m/z = 180 and 192, while the ion at m/z = 153 is a diagnostic guide for a double bond in position 5, as is the relatively low abundance of ions in the higher mass range.
The DMOX derivative of 6,9-octadecadienoate (6,9-18:2) -
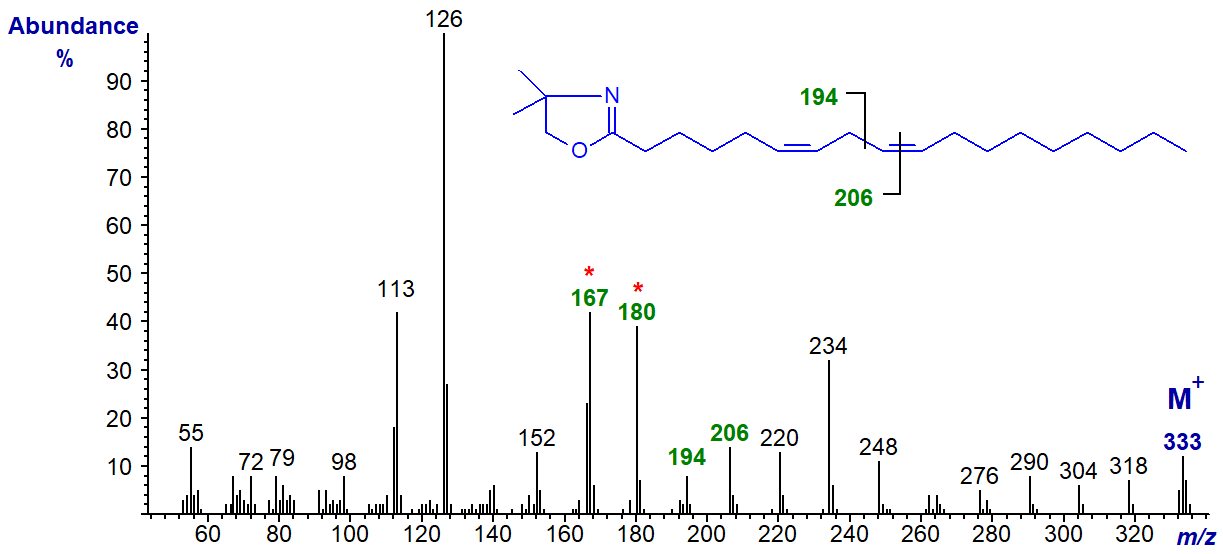
In this instance, the gap of 12 amu between m/z = 194 and 206 locates the double bond in position 9, while the fingerprint ions at m/z = 167, 180 and 194 identify that in position 6. The base ion is at m/z = 126 (as opposed to 113), as with 6-18:1
The DMOX derivative of 7,10-octadecadienoate (7,10-18:2). In this and most of the remaining isomers, the double bonds are easily located from the characteristic gaps of 12 amu. Recourse to the spectra of the monoenes simplifies the task of interpretation. Many of the following spectra are illustrated without further comment, though I have highlighted the key ions on each.
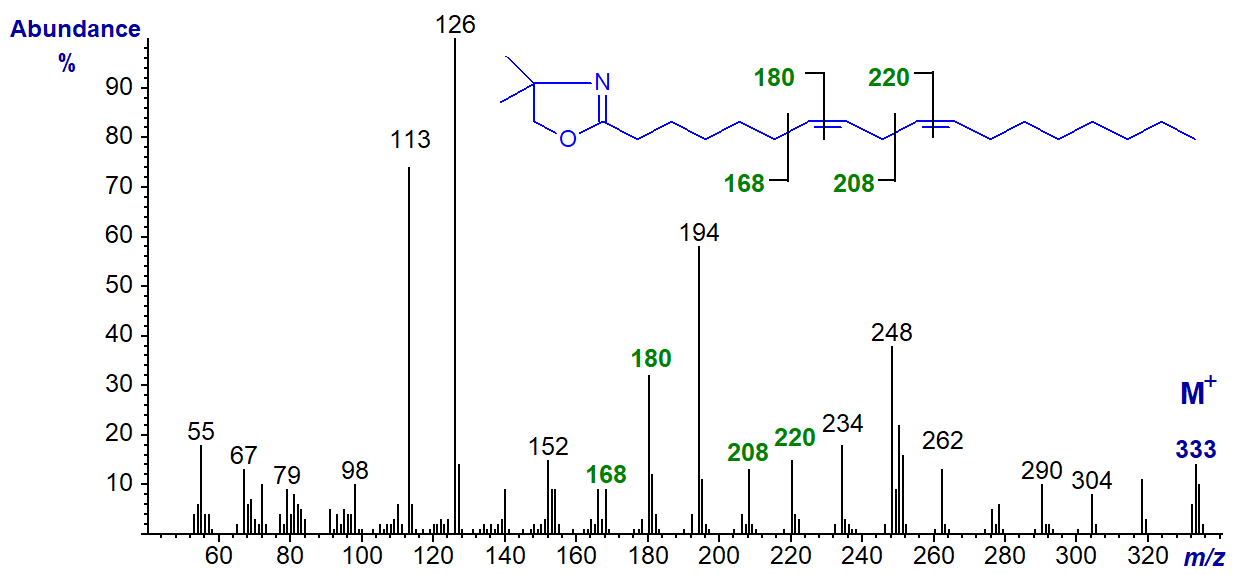
The DMOX derivative of 8,11-octadecadienoate (8,11-18:2) -
The DMOX derivative of 9,12-octadecadienoate - see start of this section.
The DMOX derivative of 10,13-octadecadienoate (10,13-18:2) -
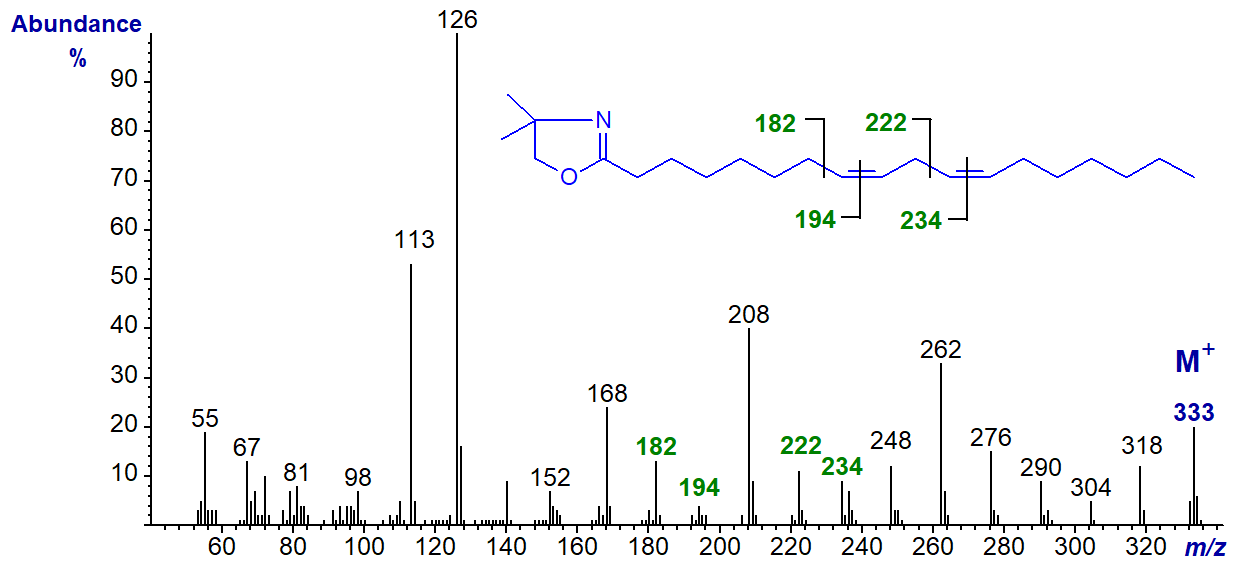
The DMOX derivative of 11,14-octadecadienoate (11,14-18:2) -
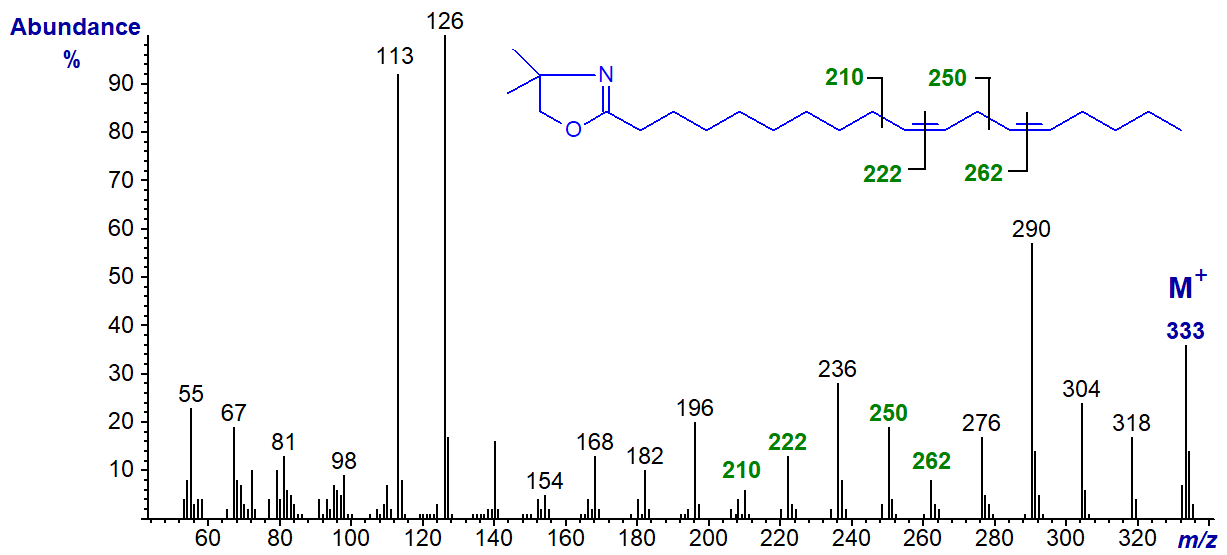
The DMOX derivative of 12,15-octadecadienoate (12,15-18:2) -
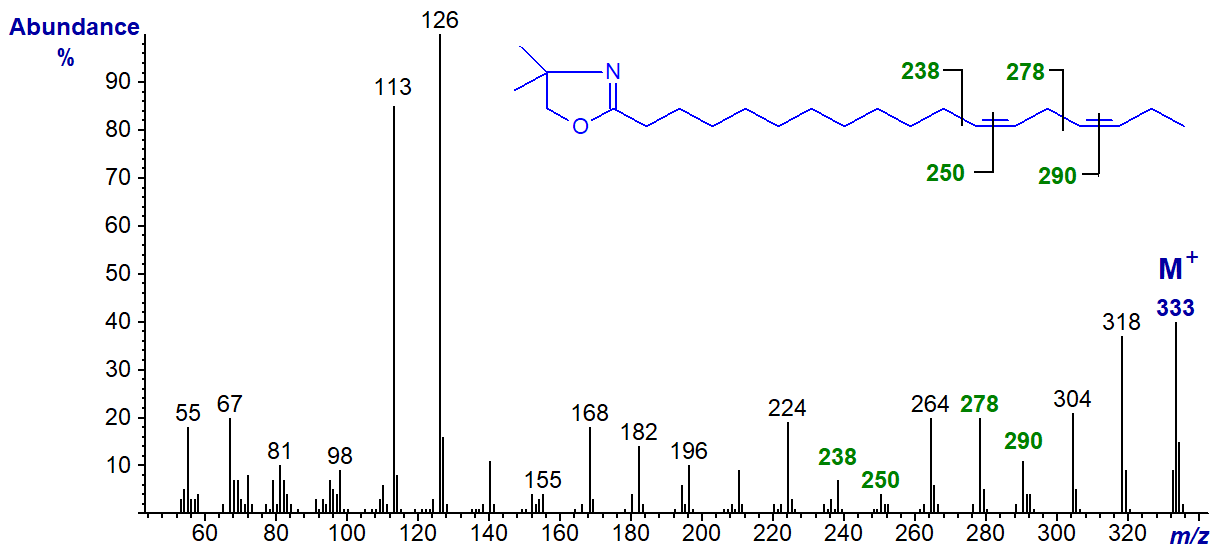
The DMOX derivative of 13,16-octadecadienoate (13,16-18:2) -
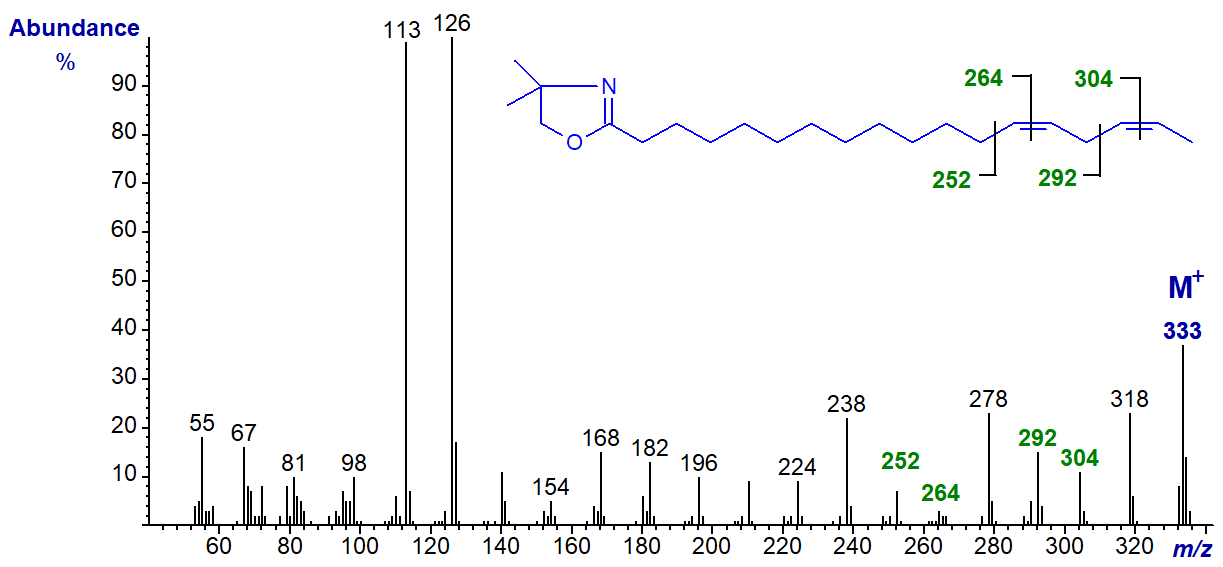
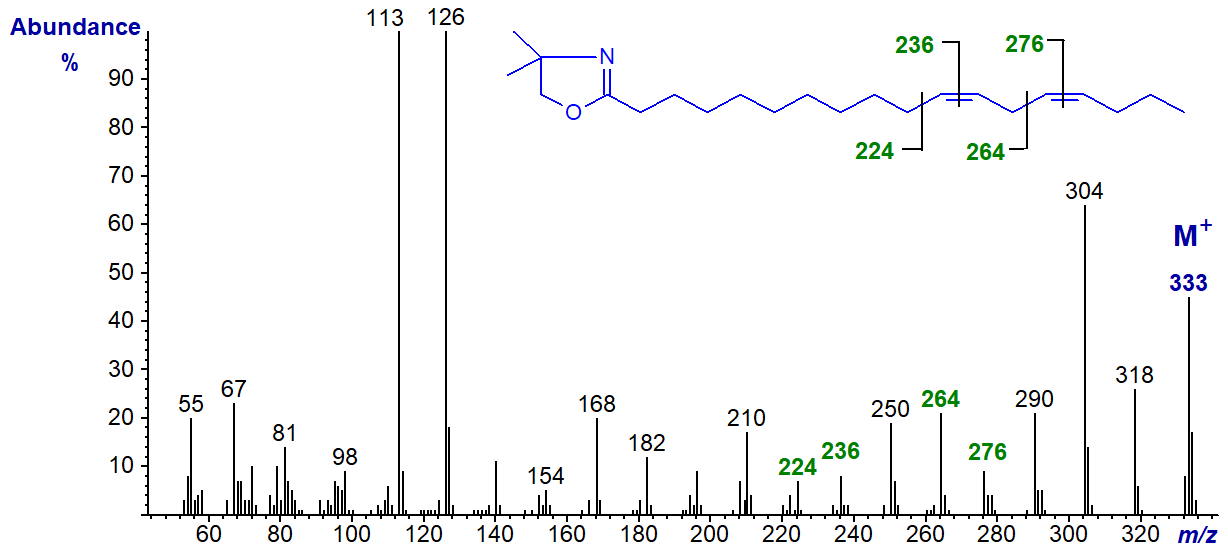
The DMOX derivative of 14,17-octadecadienoate (14,17-18:2) -
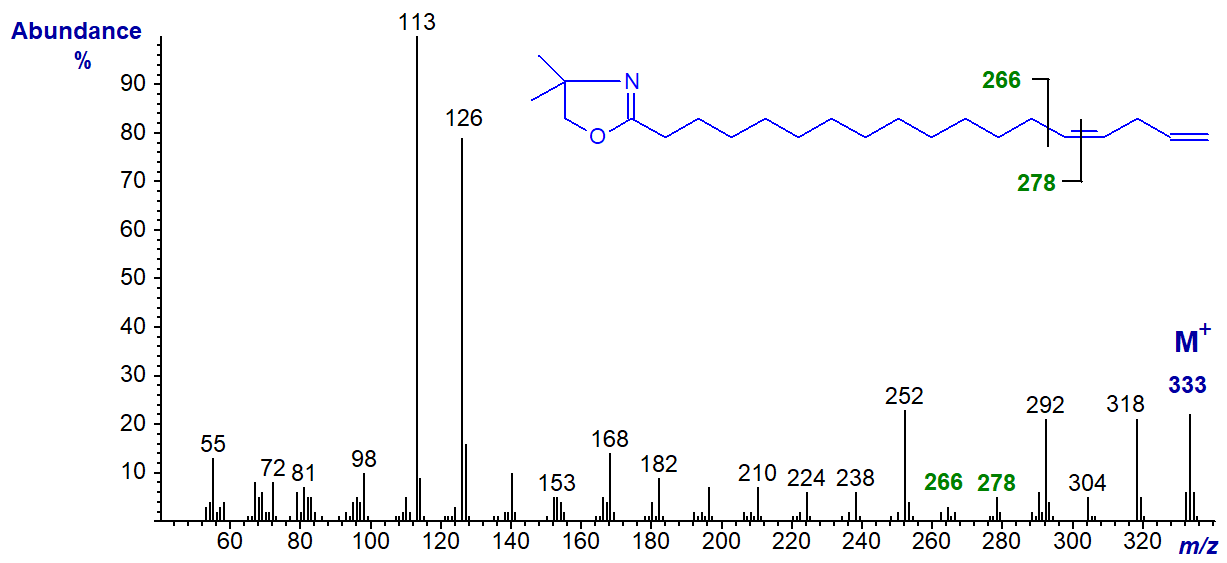
With this last isomer, note that the first double bond in position 14 can be located by the gap of 12 amu between m/z = 266 and 278, but that for the terminal double bond could be confused for one in position 16. Identification of terminal double bonds appears to be a problem with all nitrogen-containing derivatives, though there are special problems with DMOX derivatives because of a rearrangement involving loss of a methyl group from the oxazoline ring (see the Introductory web page to DMOX derivatives and Hamilton and Christie, 2000).
Some other Methylene-Interrupted Dienes
Mass spectra of DMOX derivatives of methylene-interrupted dienes of different chain-lengths are of course interpreted in the same way as the next examples show. The diagnostic ions are indicated on the spectra, and it should be evident that they are the same as on the corresponding C18 analogues above.
The DMOX derivative of 5,8-heptadecadienoate (5,8-17:2) -
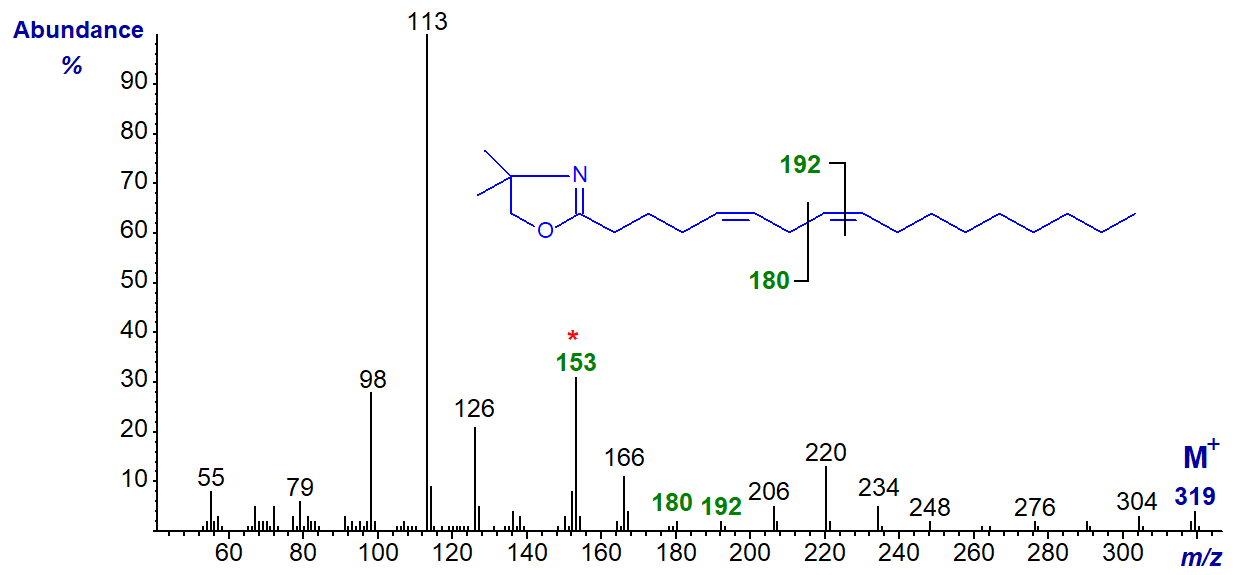
The DMOX derivative of 7,10-hexadecadienoate (7,10-16:2) -
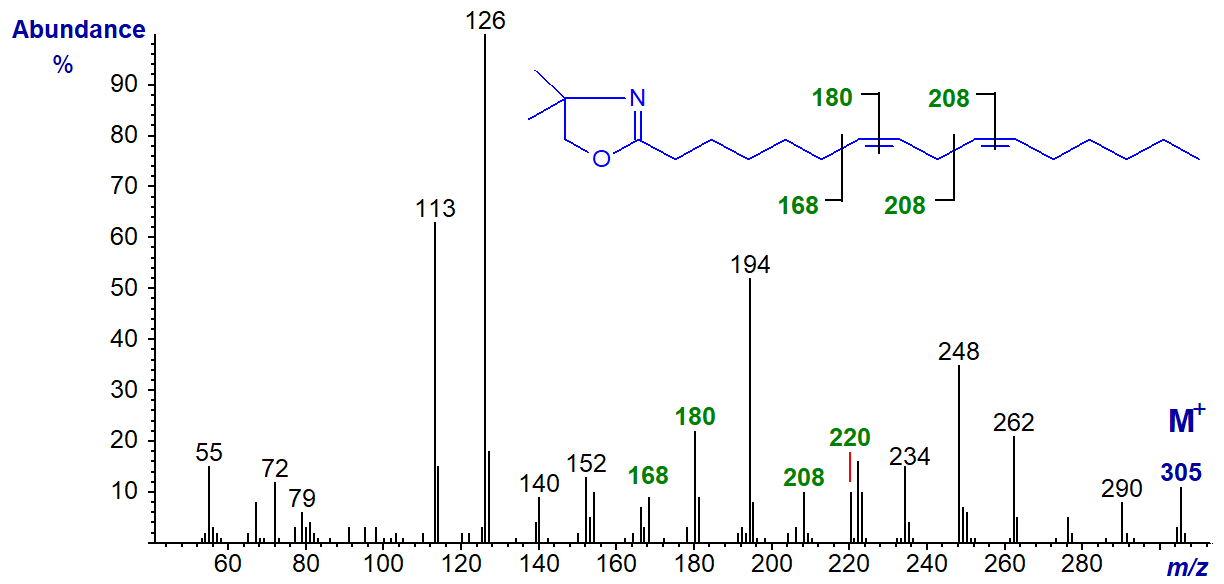
The DMOX derivative of 11,14-eicosadienoate (11,14-20:2) (Wallis and Browse, 1999) -
We have unpublished mass spectra of the DMOX derivatives of more dienoic fatty acids of this type with a range of chain-lengths, and these are available in the Archive section of these web pages but without interpretation.
References
- Christie, W.W. and Holman, R.T. Synthesis and characterization of the complete series of methylene-interrupted cis,cis-octadecadienoic acids. Chem. Phys. Lipids, 1, 407-423 (1967); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Wallis, J.G. and Browse, J. The Δ8-desaturase of Euglena gracilis: An alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch. Biochem. Biophys., 365, 307-316 (1999); DOI.
- Zhang, J.Y., Yu, Q.T., Liu, B.N. and Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom., 15, 33-44 (1988); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: November 8th, 2023 | Contact/credits/disclaimer | |
