Mass Spectrometry of Fatty Acid Pyrrolidides
Tetra-, Penta- and Hexaenoic Fatty Acids
 As was
described for dienes and trienes, the interpretation of mass spectra of pyrrolidides is analogous to that for monoenes in that it is necessary
to look for the gap of 12 amu that locates each double bond (Andersson et al., 1975).
Because it is not always easy to locate the first double bond especially, the
'fingerprint' of the appropriate monoene is a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum as I
have never yet found two positional isomers with identical spectra.
Interpretation can sometimes be aided by magnifying the ions in the high mass range relative to the base ion.
With four or more double bonds, I usually advise that the spectrum be treated as a fingerprint to be compared those of standards
because unexplained rearrangements cause complications.
I have been unable to find many illustrations of mass spectra of
pyrrolidides of the common range of polyunsaturated acids in the scientific literature.
As was
described for dienes and trienes, the interpretation of mass spectra of pyrrolidides is analogous to that for monoenes in that it is necessary
to look for the gap of 12 amu that locates each double bond (Andersson et al., 1975).
Because it is not always easy to locate the first double bond especially, the
'fingerprint' of the appropriate monoene is a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum as I
have never yet found two positional isomers with identical spectra.
Interpretation can sometimes be aided by magnifying the ions in the high mass range relative to the base ion.
With four or more double bonds, I usually advise that the spectrum be treated as a fingerprint to be compared those of standards
because unexplained rearrangements cause complications.
I have been unable to find many illustrations of mass spectra of
pyrrolidides of the common range of polyunsaturated acids in the scientific literature.
The web page on pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). It is my general impression that pyrrolidides are less useful than DMOX (or 3‑pyridylcarbinol) derivatives when trying to find the key diagnostic ions in the spectrum of an unknown polyenoic fatty acid, although they have their merits in other circumstances. We have fewer spectra of pyrrolidides available here than of DMOX derivatives, but in general the two are usually similar in the key diagnostic areas.
Tetraenoic Fatty Acids
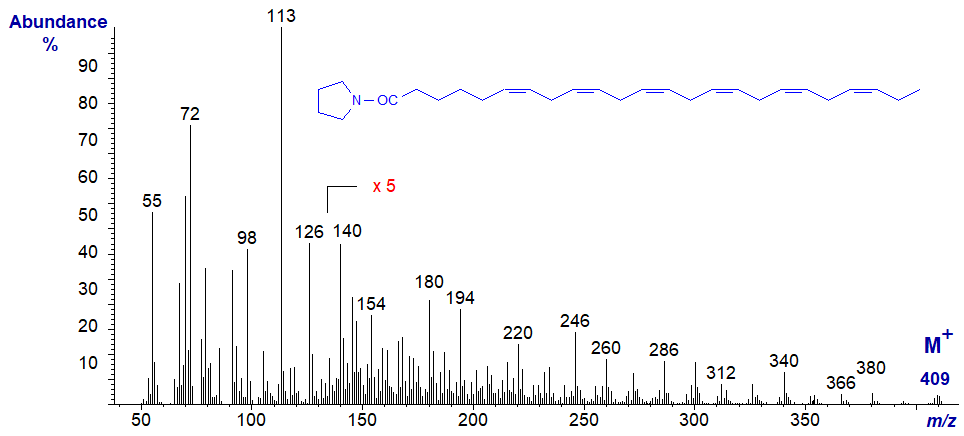
The mass spectrum of the pyrrolidide of 6,9,12,15-octadecatrienoate (stearidonate or 18:4(n-3)) from borage oil, the commercial source -
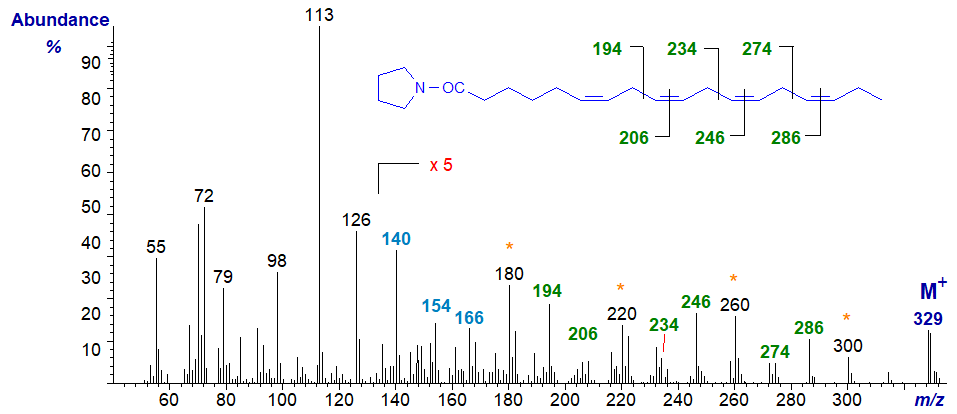
The double bond in position 6 is identified from the characteristic fingerprint ions at m/z = 140, 154 and 166, while the double bonds in positions 9, 12 and 15 are recognized by the gaps of 12 amu between m/z = 194 to 206, 234 to 246, and 274 to 286, respectively. On the other hand as with trienes, it is often easier to locate the gaps of 40 amu for the double bond plus adjacent methylene group, in this instance between m/z = 180 to 220 to 260 to 300 (or 194 to 234 to 274) as indicated on the spectrum (see the discussion of the corresponding DMOX derivatives).
The mass spectrum of arachidonoyl pyrrolidine (5,8,11,14-20:4 or 20:4(n-6) - the essential fatty acid from animal tissues) follows -
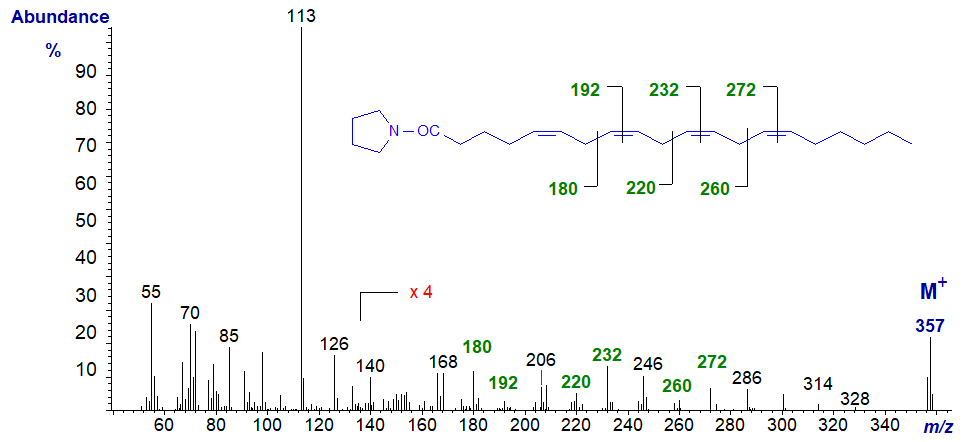
Gaps of 12 amu between m/z = 180 and 192, 220 and 232, and 260 and 272, locate the double bonds in positions 8, 11 and 14, respectively. That in position 5 must be inferred, as the expected diagnostic ion at m/z = 153 is submerged among many small ions (Valicenti et al., 1978).
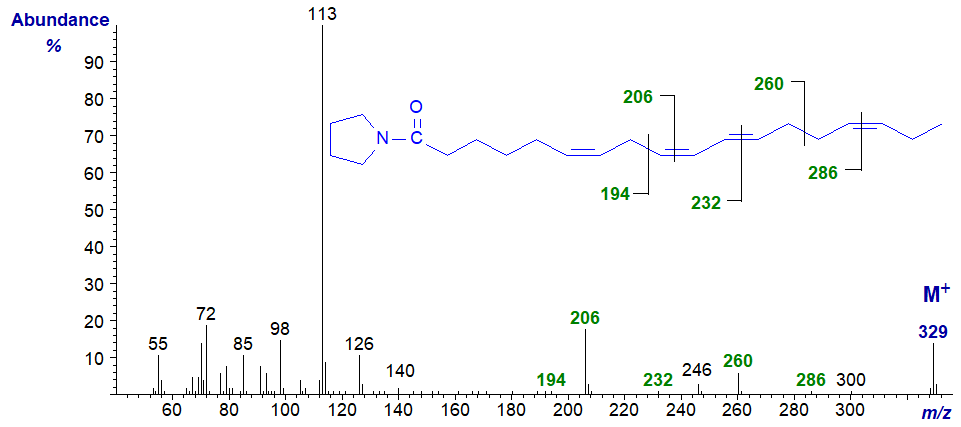
A further useful mass spectrum of a tetraene from animal tissues is that of 7,10,13,16-docosatetraenoate (22:4(n-6) or adrenate) -
The spectrum is too confused to serve as an absolute guide to locate double bond position, but at least is a useful fingerprint. While the expected ions are indeed present, other ions could confound any interpretation without further evidence.
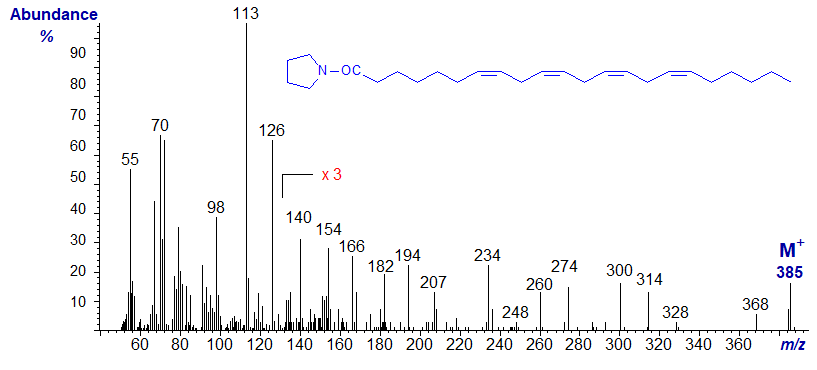
We have the spectrum of one other interesting tetraene on file, with two double bonds in conjugation as well as a bis-methylene-interrupted double bonds, produced by microbial action on stearidonic acid (Hennessy et al., 2012), i.e., the pyrrolidide of 6,9,11,15-octadecatetraenoate (6,9,11,15-18:4) -
It is not especially distinctive in terms of structural information, and the few diagnostic ions are marked. Comparison with the spectrum of the related triene 6,9,11‑octadecatrienoate should prove valuable. As with the latter, additional evidence was required to prove the structure. Note that the spectrum is appreciable different from that of the 6,9,12,15‑isomer in Figure 1.
Pentaenoic Fatty Acids
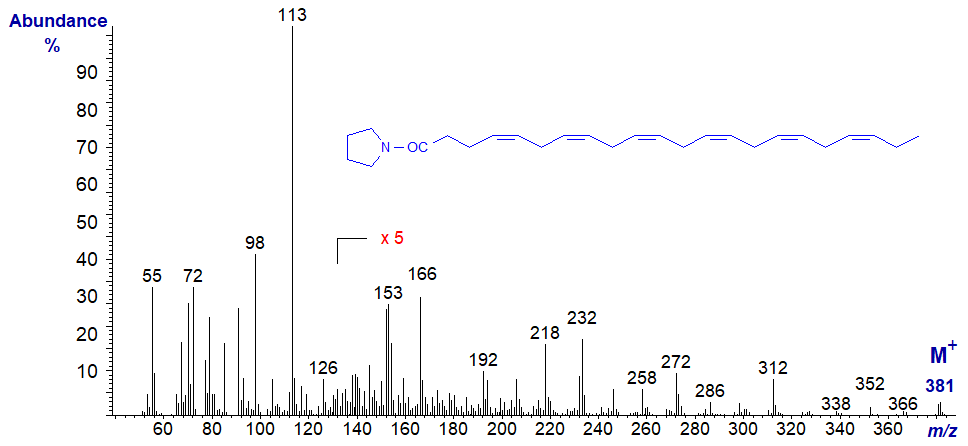
The following spectra are all best considered as fingerprints, without trying to pick out the key diagnostic ions, which are usually confused in all polyenes. No interpretation is offered therefore, and the spectra are left to speak for themselves. Although the molecular ions are small, they are easier to identify than in the corresponding methyl esters.
Mass spectrum of the pyrrolidide of 5,8,11,14,17-eicosapentaenoate (20:5(n-3) or EPA) -
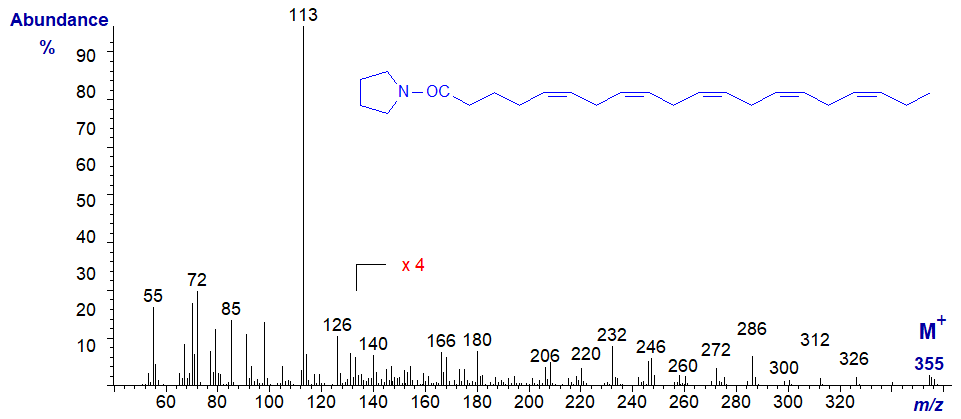
Mass spectrum of the pyrrolidide of 4,7,10,13,16-docosapentaenoate (or 22:5(n-6)) -
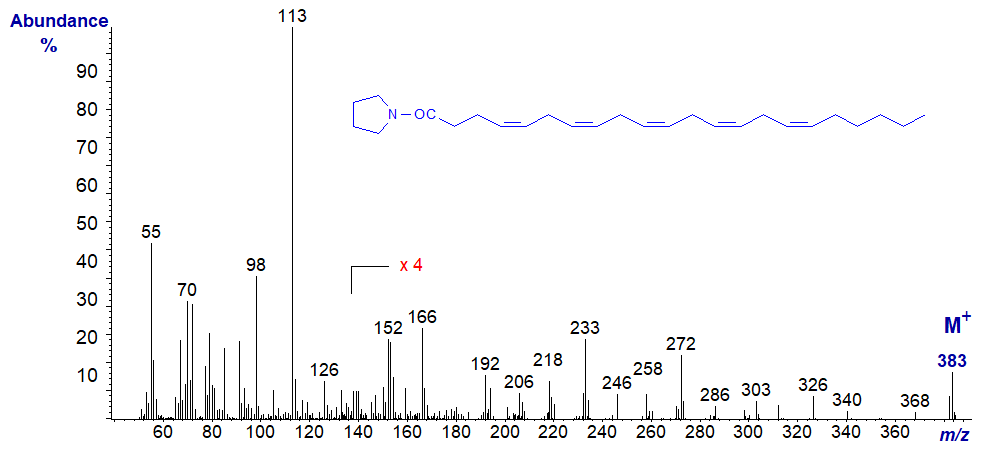
Mass spectrum of the pyrrolidide of 7,10,13,16,19-docosapentaenoate (or 22:5(n-3)) -
Hexaenoic Fatty Acids
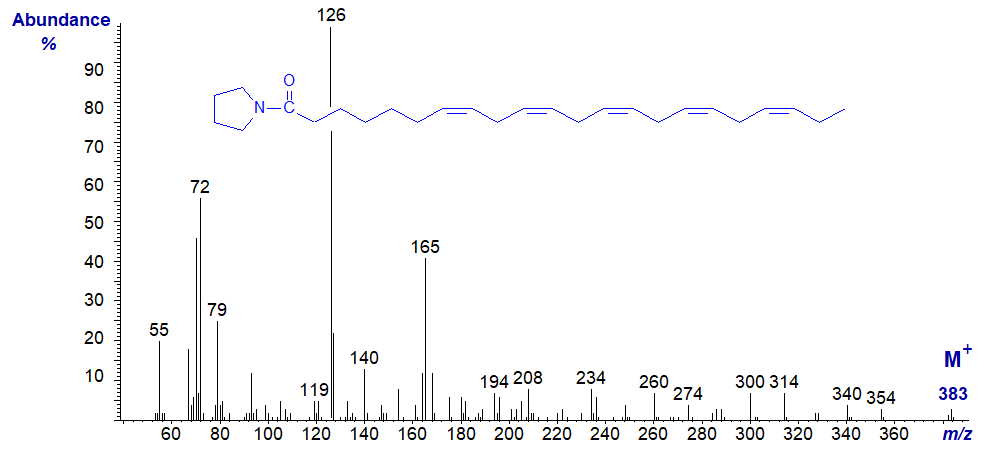
Mass spectrum of the pyrrolidide of 4,7,10,13,16,19-docosahexaenoate (22:6(n-3) or DHA) -
Mass spectrum of the pyrrolidide of 6,9,12,15,18,21-tetracosahexaenoate (24:6(n-3)) -
Spectra of a few more pyrrolidine derivatives of polyunsaturated fatty acids are available on our Archive pages, but without interpretation.
References
- Andersson, B.A., Christie, W.W. and Holman, R.T. Mass spectrometric determination of positions of double bonds in polyunsaturated fatty acid pyrrolidides. Lipids, 10, 215-219 (1975); DOI.
- Hennessy, A.A., Barrett, E., Ross, R.P., Fitzgerald, G.F., Devery, R. and Stanton, C. The production of conjugated α-linolenic, γ‑linolenic and stearidonic acids by strains of Bifidobacteria and Propionibacteria. Lipids, 47, 313-327 (2012); DOI.
- Valicenti, A.J., Chapman, C.J., Holman, R.T. and Chipault, J.R. Mass spectrometry identification of C20 fatty acids in bovine lens using the pyrrolidide derivative. Lipids, 13, 190-194 (1978); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
