Mass Spectrometry of Fatty Acid Pyrrolidides
Trienoic Fatty Acids
 As was
described for dienes, the interpretation of mass spectra of pyrrolidides is similar to that for monoenes in that it is necessary
to look for the gap of 12 amu that locates each double bond.
As it is not always easy to locate the first double bond especially, the 'fingerprint' of the appropriate
monoene is then a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum, as I
have never yet found two positional isomers with identical spectra.
With four or more double bonds, I usually advise that the spectrum be treated as a fingerprint to be compared those of standards.
I have been unable to find many illustrations of mass spectra of pyrrolidides of the common range of polyunsaturated acids in the
scientific literature.
As was
described for dienes, the interpretation of mass spectra of pyrrolidides is similar to that for monoenes in that it is necessary
to look for the gap of 12 amu that locates each double bond.
As it is not always easy to locate the first double bond especially, the 'fingerprint' of the appropriate
monoene is then a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum, as I
have never yet found two positional isomers with identical spectra.
With four or more double bonds, I usually advise that the spectrum be treated as a fingerprint to be compared those of standards.
I have been unable to find many illustrations of mass spectra of pyrrolidides of the common range of polyunsaturated acids in the
scientific literature.
The web page on pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). It is my general impression that pyrrolidides are less useful than DMOX (or 3-pyridylcarbinol) derivatives when trying to find the key diagnostic ions in the spectrum of an unknown polyenoic fatty acid, although they have their merits in other circumstances. We have fewer spectra of pyrrolidides available here than of DMOX derivatives, but in general the two are usually comparable in the key diagnostic areas.
Methylene-Interrupted Trienoic Fatty Acids
With higher polyunsaturated fatty acids, the picture becomes even less clear than with mono- and dienes, as there is more noise around the diagnostic ions that hampers recognition. Thus, the earlier rule was extended by Andersson et al. (1975) as -
"The presence of a peak relatively more intense than the peak clusters which flank it and which are involved in probable intervals of 12 amu indicates the presence of a methylene-interrupted system. If the prominent peak contains m carbons of the fatty acid residue, the methylene carbon in the molecule is at position m+1".
Starting with the mass spectra of the two trienes encountered most often, that of the pyrrolidine derivative of 6,9,12-octadecatrienoate (or γ‑linolenate or 18:3(n-6)) (Das Gupta et al., 1992) is -
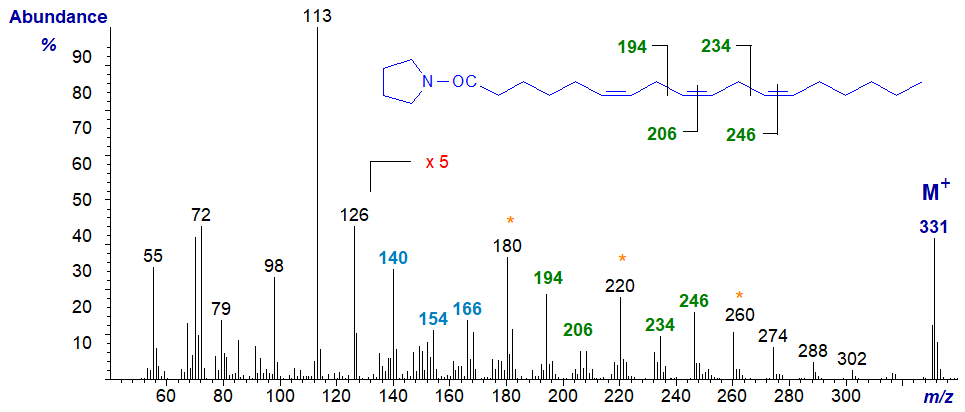
The ions in the higher mass range have all been magnified five-fold relative to the base ion as an aid to identification. The double bond in position 6 is best recognized via the spectrum of the 6-monoene by the characteristic ions at m/z = 140, 154 and 166 (see our web page on pyrrolidides of monoenes). Those in positions 9 and 12 are located by the gaps of 12 amu between 194 and 206, and between 234 and 246, respectively. On the other hand, it is often easier to locate the gaps of 40 amu for the double bond plus adjacent methylene group, in this instance between m/z = 180 to 220 to 260 as indicated on the spectrum (see the discussion of the corresponding DMOX derivatives here...).
The mass spectrum of the pyrrolidine derivative of the ubiquitous 9,12,15-octadecatrienoate (or α-linolenate or 18:3(n-3)) (Andersson et al., 1975) is -
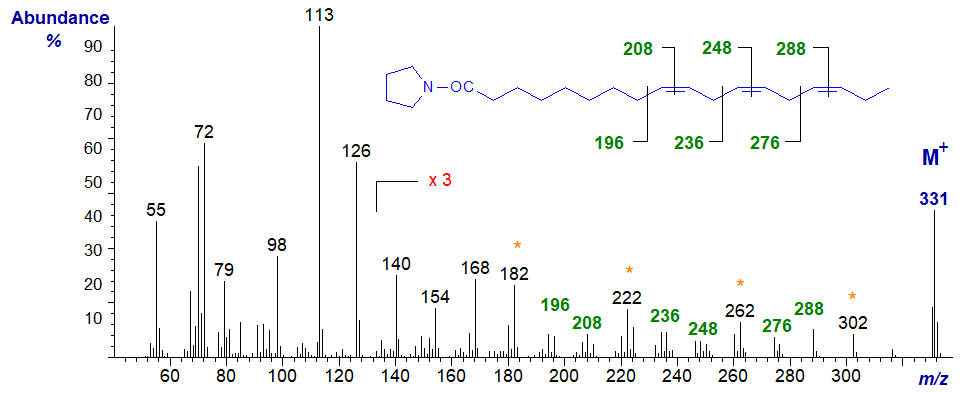
The gaps of 12 amu between m/z = 196 and 208, 236 and 248, and 276 and 288, define the double bonds in positions 9, 12 and 15, respectively, but from a practical standpoint, it is just as well we have the standard spectrum to consult. It helps that for methylene-interrupted fatty acids, the equivalent diagnostic ions for each double bond should be 40 amu apart (m/z = 196 to 236 to 276, and 208 to 248 to 288), although the gaps of 40 amu between m/z = 182 to 222 to 262 to 302 are a better practical guide.
The mass spectrum of the pyrrolidine derivative of 9,12,15-hexadecatrienoate (or 16:3(n-1)) from a genetically modified yeast -
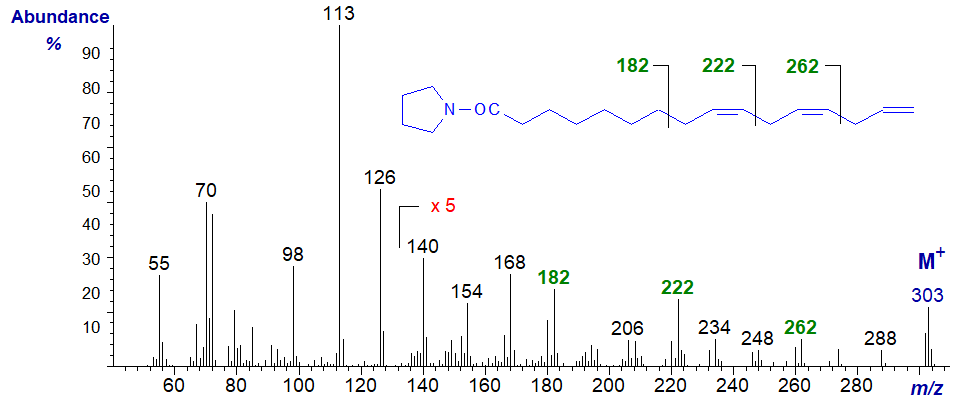
Although the gaps of 12 amu are indeed present in this spectrum, the gaps of 40 amu highlighted in the previous spectrum and again here are easier to use diagnostically.
The mass spectrum of the pyrrolidine derivative of 5,8,11-eicosatrienoate (or 20:3(n-9) or Mead's acid), a fatty acid that assumes importance in essential fatty acid deficiency, is -
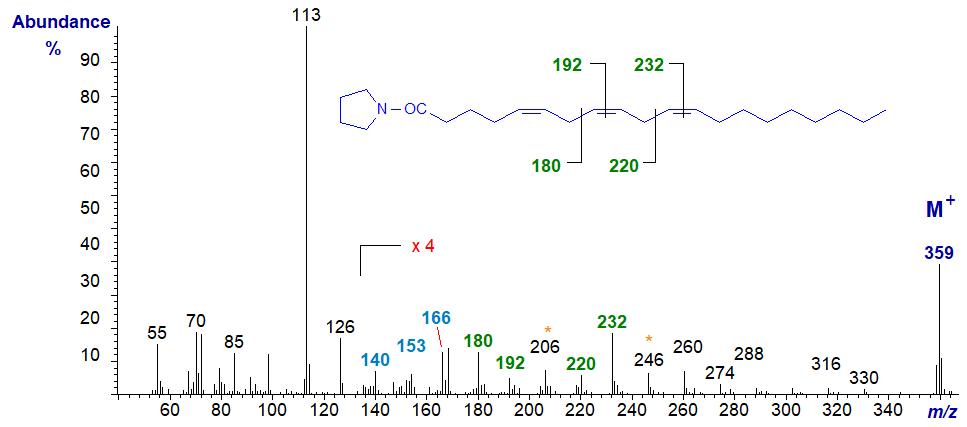
The double bond in position 5 is located by the characteristic fingerprint of ions at m/z = 140, 153 and 166 (a small but significant difference from a 6-isomer), while those in positions 8 and 11 are defined by the gaps of 12 amu between m/z = 180 and 192, and 220 and 232, respectively (Valicenti et al., 1978).
The mass spectrum of the pyrrolidine derivative of 8,11,14-eicosatrienoate (or 20:3(n-6)) is -
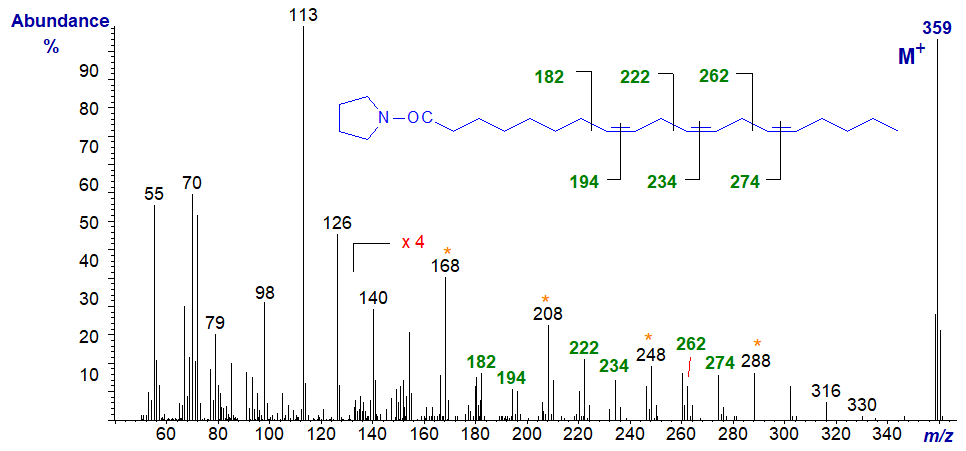
The important diagnostic ions denote the gaps of 12 amu between m/z = 182 and 194, 222 and 234, and 262 and 274, for the double bonds in positions 8, 11 and 14, respectively, although the gaps of 40 amu as highlighted on the spectrum are informative.
Trienoic Fatty Acids with Conjugated Double Bond Systems
We spectra from only one fatty acid with a conjugated double bond system on file, i.e., the pyrrolidide of 6,9,11-octadecatrienoate (6,9,11‑18:3), prepared by microbial action on γ-linolenic acid (Hennessy et al., 2012) -
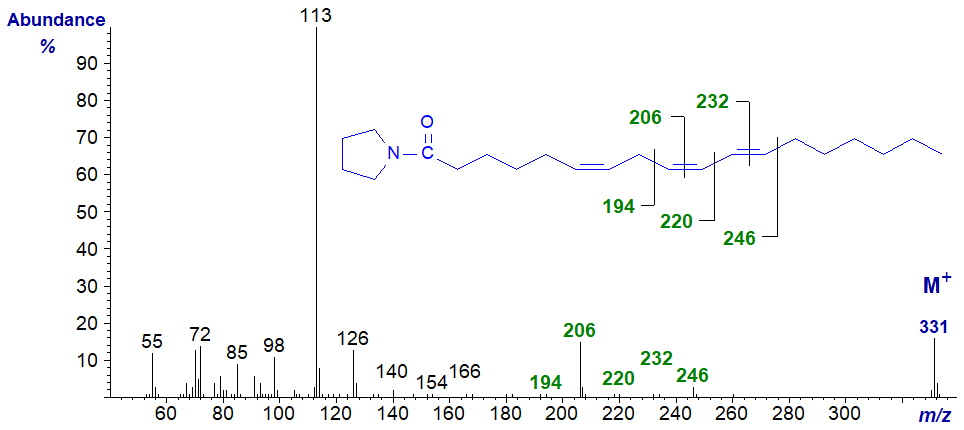
The key diagnostic ions are marked. In truth, assignments of the double bond positions would have been difficult if we had not had access to further experimental data.
Bis-and Polymethylene-Interrupted Trienoic Fatty Acids
A few trienoic acids with bis-methylene-interrupted double bond systems are known, especially from gymnosperms and marine invertebrates such as sponges. 5,9,12‑Octadecatrienoic (pinolenic) acid is found in the lipids of pine species, and the mass spectrum of its pyrrolidide derivative is -
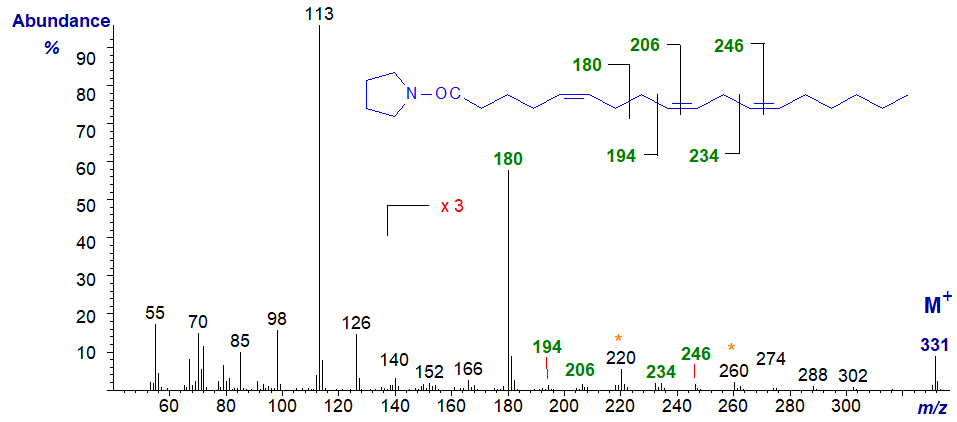
The distinctive feature is an ion at m/z = 180 formed by cleavage at the centre of the bis-methylene-interrupted double bond system (cf., the spectra of 5,9-16:2 and of the DMOX derivative). The double bond in position 5 would otherwise not be easy to locate from first principles, but those in positions 9 and 12 are confirmed by the gaps of 12 amu between m/z = 194 and 206, and 234 and 246, respectively. Note the gaps of 40 amu here.
Rather more unusual is a fatty acid with two bis-methylene-interrupted double bond systems, and the mass spectrum of the pyrrolidide of 5,9,13‑eicosatrienoate (5,9,13-20:3) from a sponge is -
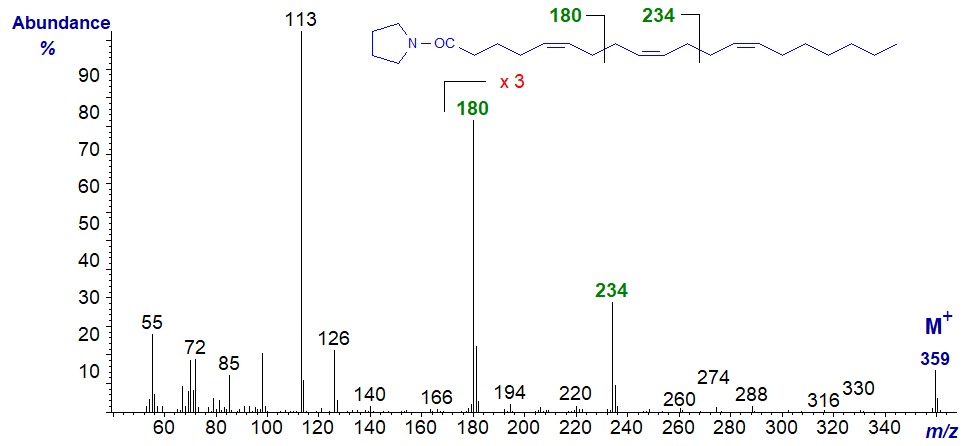
The ion at m/z = 180 for cleavage between carbons 7 and 8, seen in the previous spectrum, is again evident, while the distinctive ion at m/z = 234 represents cleavage between carbons 11 and 12 (data first published by Carballeira and Medina, 1994).
Trienoic acids with more than two methylene groups between double bonds are less common in nature, but 3,9,12-octadecatrienoate is occasionally found in seeds of Chrysanthemum and related species, although the double bond in position 3 is usually of the trans rather than the cis configuration as here. With some reservation, its pyrrolidide may have the spectrum -
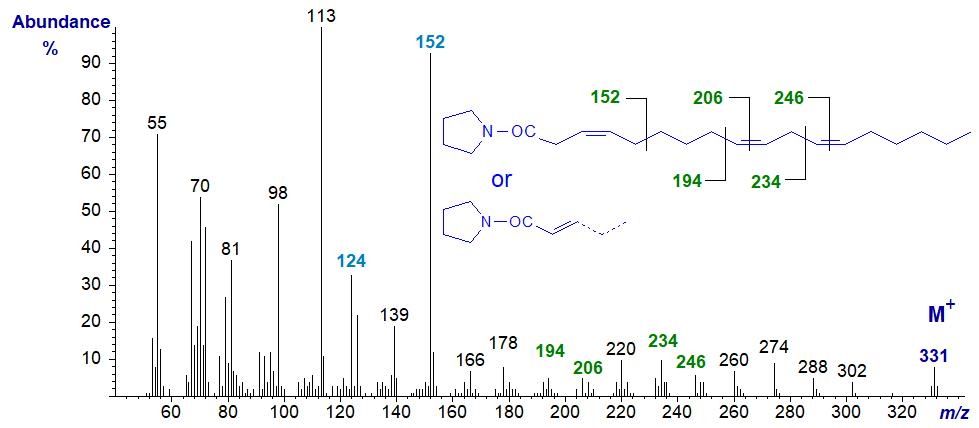
The double bonds in positions 9 and 12 are located as marked. On the other hand, with further experience, it now appears that the double bond in position 3 has migrated to position 2 during the derivatization step, so that the above is probably the spectrum of the 2,9,12-isomer in fact, not that of the original fatty acid. The ions at m/z = 124 and 152 are characteristic of 2-isomers.
5,11,14-Eicosatrienoate (or 5,11,14-20:3) is found in seeds from some species of Gymnosperms and the mass spectrum of its pyrrolidide is -
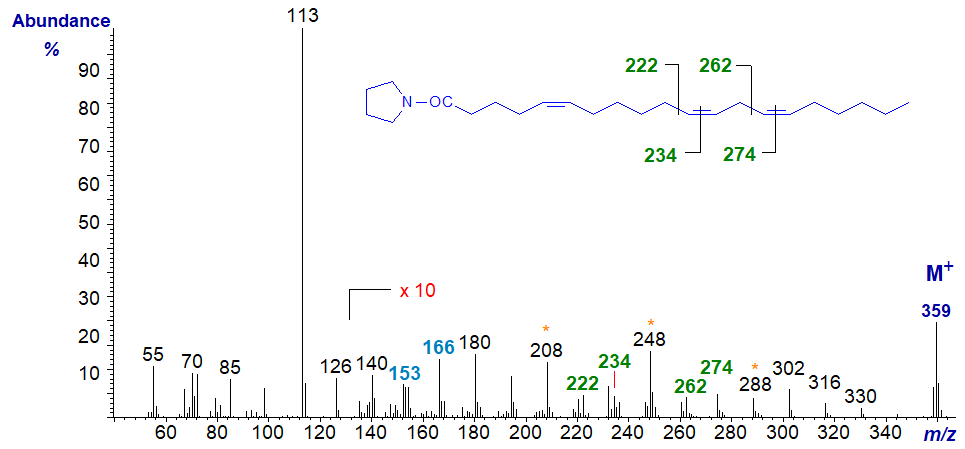
As is usual with double bonds in position 5, the ions in the higher mass range are of rather low abundance and a ten-fold magnification is needed to see them clearly. The double bonds in the 11 and 14 positions are located by the gaps of 12 amu between m/z = 222 and 234, and 262 and 274, respectively, but we need the fingerprint ions at m/z = 153 and 166 for the double bond in position 5.
Our last example is the spectrum of a fatty acid with both a bis-methylene-interrupted double bond system and a (very) isolated double bond from a sponge, i.e., of the pyrrolidide of 5,9,19-octacosatrienoate (5,9,19-28:3) -
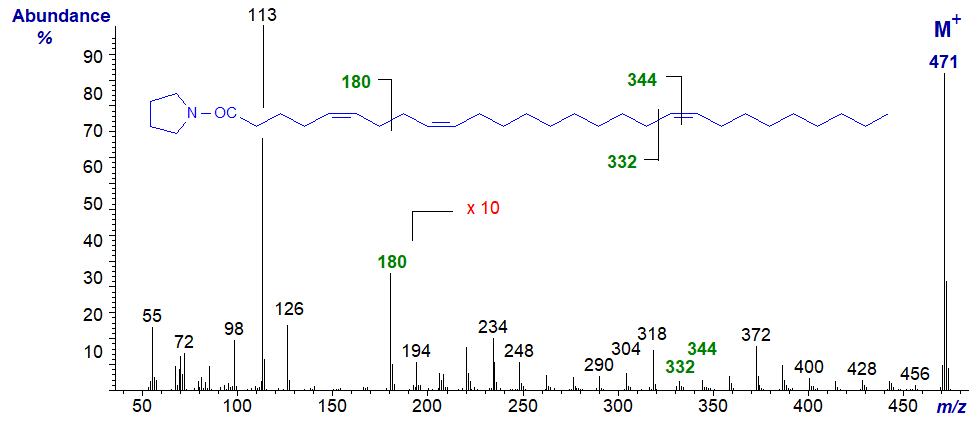
Even in such an unusual fatty acid, the double bonds are clearly located as marked.
Spectra of a few more pyrrolidine derivatives of trienoic fatty acids are available on our Archive pages, but without interpretation.
References
- Andersson, B.A., Christie, W.W. and Holman, R.T. Mass spectrometric determination of positions of double bonds in polyunsaturated fatty acid pyrrolidides. Lipids, 10, 215-219 (1975); DOI.
- Carballeira, N.M. and Medina, J.R. New Δ5,9 fatty acids in the phospholipids of the sea anemone Stoichactis helianthus. J. Nat. Prod., 57, 1688-1695 (1994); DOI.
- Dasgupta, A., Banerjee, P. and Malik, S. Use of microwave irradiation for rapid transesterification of lipids and accelerated synthesis of fatty acyl pyrrolidides for analysis by GC-MS: study of fatty acid profiles of olive oil, evening primrose oil, fish oils and phospholipids from mango pulp. Chem. Phys. Lipids, 62, 281-291 (1992); DOI.
- Hennessy, A.A., Barrett, E., Ross, R.P., Fitzgerald, G.F., Devery, R. and Stanton, C. The production of conjugated α-linolenic, γ‑linolenic and stearidonic acids by strains of Bifidobacteria and Propionibacteria. Lipids, 47, 313-327 (2012); DOI.
- Valicenti, A.J., Chapman, C.J., Holman, R.T. and Chipault, J.R. Mass spectrometry identification of C20 fatty acids in bovine lens using the pyrrolidide derivative. Lipids, 13, 190-194 (1978); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
