Mass Spectrometry of DMOX Derivatives
Tetra- to Hexaenoic Fatty Acids
As with trienes, the mass spectra of DMOX derivatives of further polyenoic fatty acids permit location of the double bonds, but less easily than those of monoenes and dienes. The diagnostic ions often occur at the centres of clusters and do not stand out well, although different isomers tend to have very different spectra so that characterization is possible when spectra of authentic fatty acids can be compared. The principles described in the earlier documents in this series apply, so we are in general looking for the diagnostic gaps of 12 amu between significant ions. When the first double bond is close to the carboxyl group, the general fragmentation rule for double bond location is no longer appropriate, and the 'fingerprint' spectrum for the relevant monoene derivative is an invaluable guide to its identification.
Few model compounds are available, so most of the following spectra have been obtained from analyses of natural products as part of our research programme, and fatty acids with a variety of chain-lengths are described. As with other nitrogen-containing derivatives, the molecular ion is always clearly delineated, in contrast to the mass spectra of methyl esters. References are listed when we are aware of prior formal publication of spectra in the scientific literature.
Methylene-Interrupted Tetraenoic Fatty Acids
The mass spectrum of the DMOX derivative of 6,9,12,15-octadecatetraenoate (stearidonate or 18:4(n-3)), a minor component of fish and some seed oils, is illustrated first -
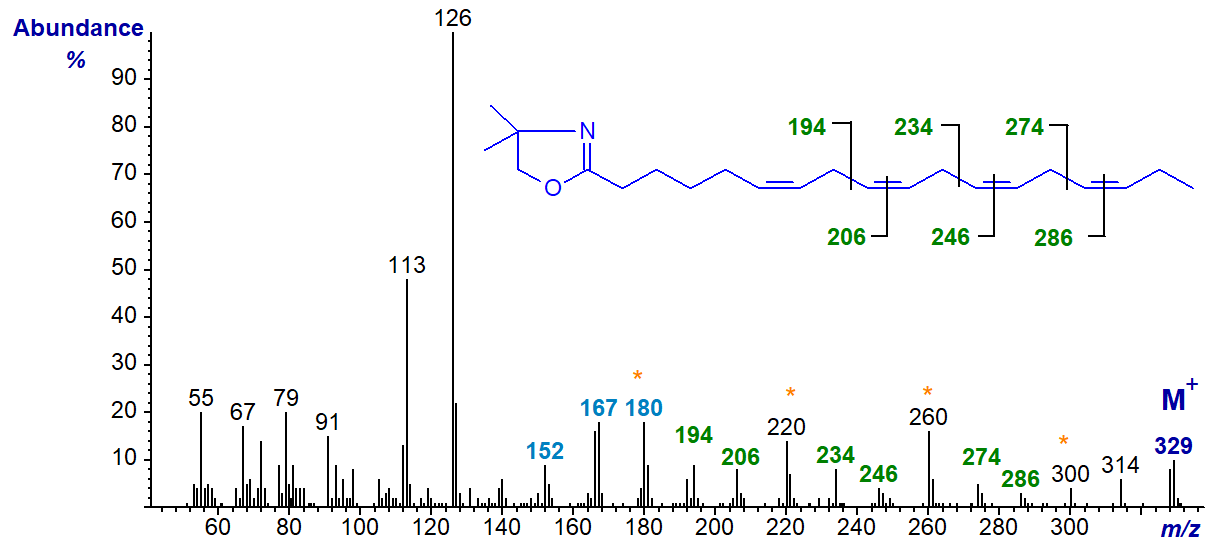
The double bond in position 6 is recognized by the characteristic fingerprint of ions at m/z = 152, 167 and 180 (DMOX derivatives of monoenoic fatty acids). The remainder are located by the gaps of 12 amu between m/z = 194 and 206, 234 and 246, and 274 and 286, i.e., for positions 9, 12 and 15, respectively, as can be deduced by extrapolation from our document on monoenes (Yu, Q.T. et al., 1989; Sayanova, O. et al., 1997). As with trienoic DMOX derivatives, the gaps of 40 amu between m/z = 194, 234 and 274, or between m/z = 206, 246 and 286, or best of all between m/z = 180, 220, 260 and 300, are useful diagnostic guides for the last three double bonds. Indeed, these gaps of 40 amu can sometimes be easier to detect than those of 12 amu.
The DMOX derivative of 8,11,14,17-octadecatetraenoate or 18:4(n-1) - a minor component of fish oils -
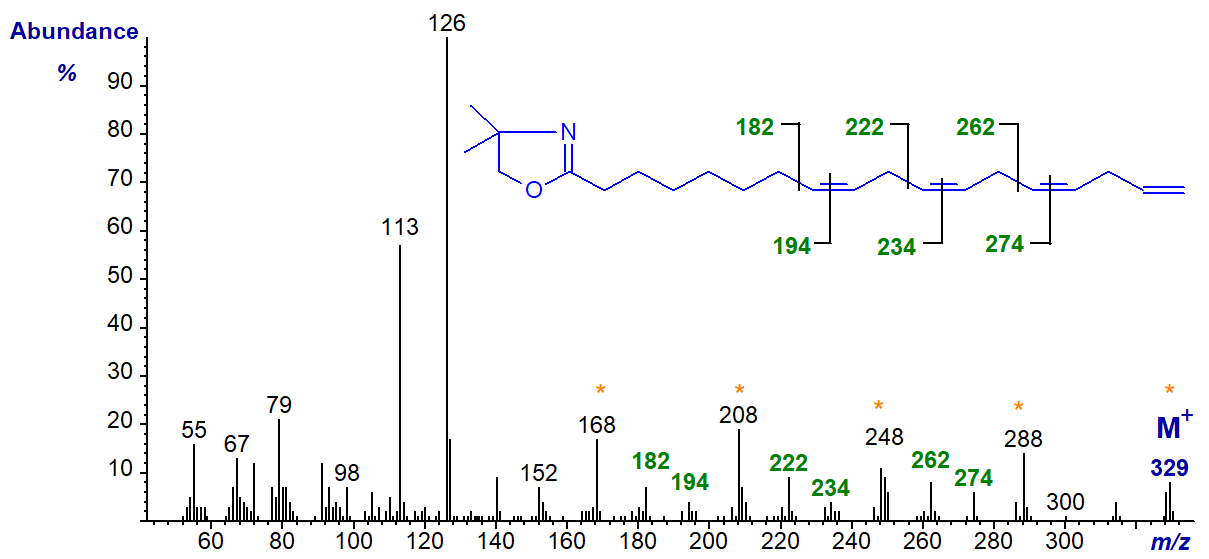
The double bonds in positions 8, 11 and 14 can be recognized by the appropriate gaps of 12 amu, as indicated. That in position 17 can only be inferred, although the gaps of 40 amu (m/z = 168 to 208 to 248 to 288 to 329) provide some additional evidence. However, terminal double bonds are not easily recognized with pyrrolidides and 3‑pyridylcarbinol ('picolinyl') esters either.
The DMOX derivative of 5,8,11,14-eicosatetraenoate (20:4(n-6) or arachidonate), the important essential fatty acid and precursor of so many eicosanoids -
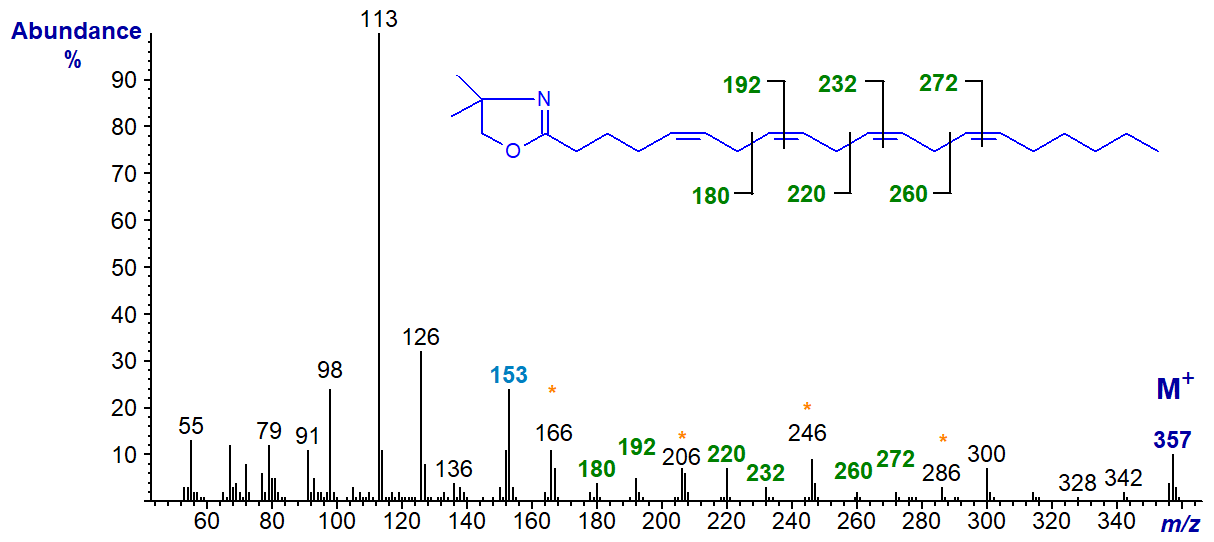
The double bond in position 5 is confirmed by the fingerprint ion at m/z = 153, together with the relative abundance of the ion at m/z = 113, while the remainder are located by the gaps of 12 or 40 amu as indicated (Zhang, J.Y. et al., 1988; Yu, Q.T. et al., 1989; Wolff, R.L. et al., 1999). The same diagnostic ions are present in the spectrum of the DMOX derivative of 5,8,11,14-18:4 or 18:4(n-4) in our Archive pages.
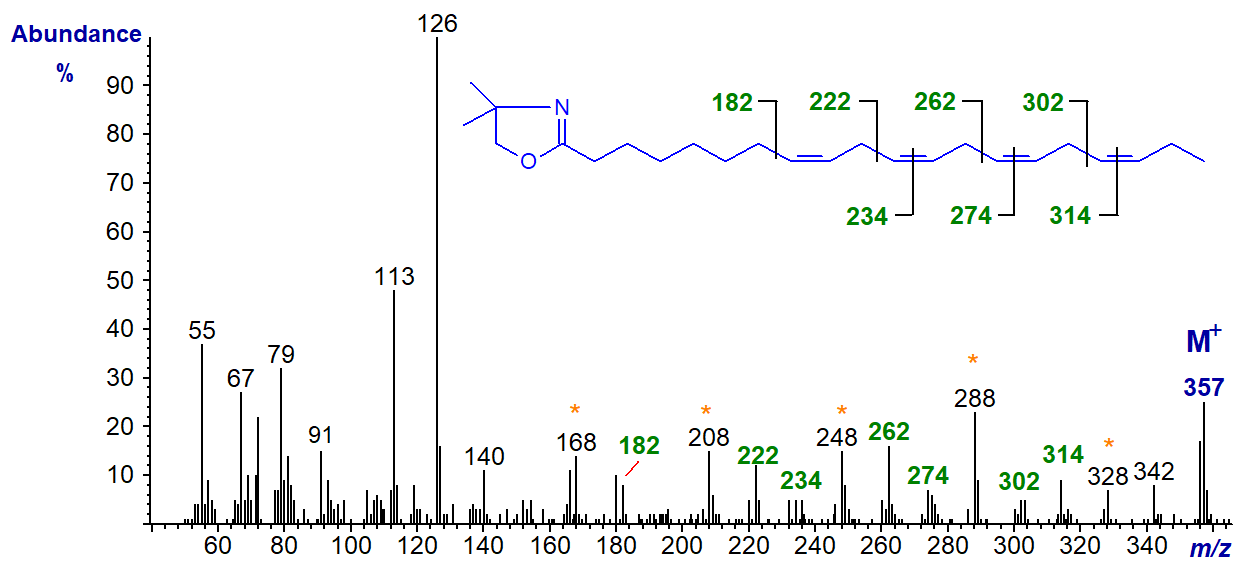
The DMOX derivative of 8,11,14,17-eicosatetraenoate (20:4(n-3)) (Yu, Q.T. et al., 1989; Luthria and Sprecher, 1993). The diagnostic ions are marked.
We have the mass spectra of the DMOX derivatives of several more methylene-interrupted tetraenes, including C16 and C22 isomers, on file in the Archive Section of the web pages, but without interpretation.
Tetraenoic Fatty Acids with Conjugated Double Bonds
We were unable to prepare the DMOX derivative of the common conjugated tetraene parinaric acid, possibly because it isomerized on derivatization, but we do have the spectrum of a fatty acid derived by microbial action on stearidonic acid with three double bonds in conjugation (stereochemistry not defined) and one isolated double bond, i.e., 6,8,10,15-octadecatetraenoate (Hennessy, A.A. et al., 2012) -
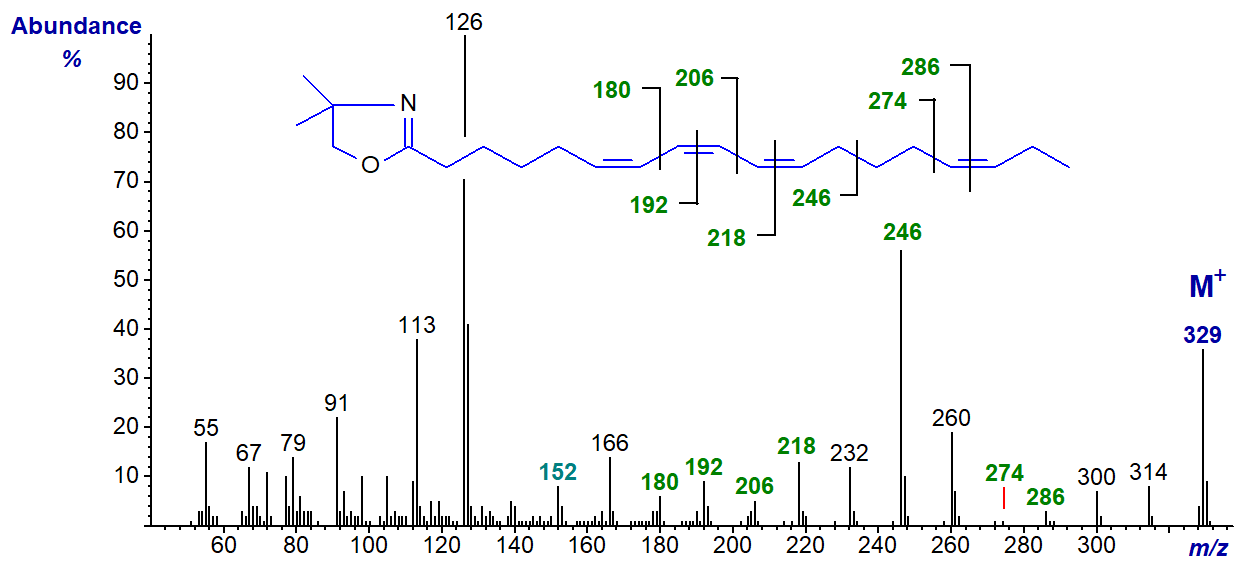
The small ion at m/z = 152 is a fingerprint ion for the double bond in position 6, although this would not be convincing if other evidence were not available; the other double bonds are easily located by the ions marked. The large ion at m/z = 246 for a fragmentation beta to the conjugated double bond system is comparable to a distinctive ion in the spectrum of the DMOX derivative of 9-cis,11-trans,15-cis-octadecatrienoate.
Bis- and Polymethylene-Interrupted Tetraenoic Fatty Acids
We have a few relevant spectra on file garnered from natural sources, and the DMOX derivative of 5,11,14,17-eicosatetraenoate (5,11,14,17-20:4 or juniperonate) from the seed oil of a gymnosperm (Berdeau and Wolff, 1996) is -
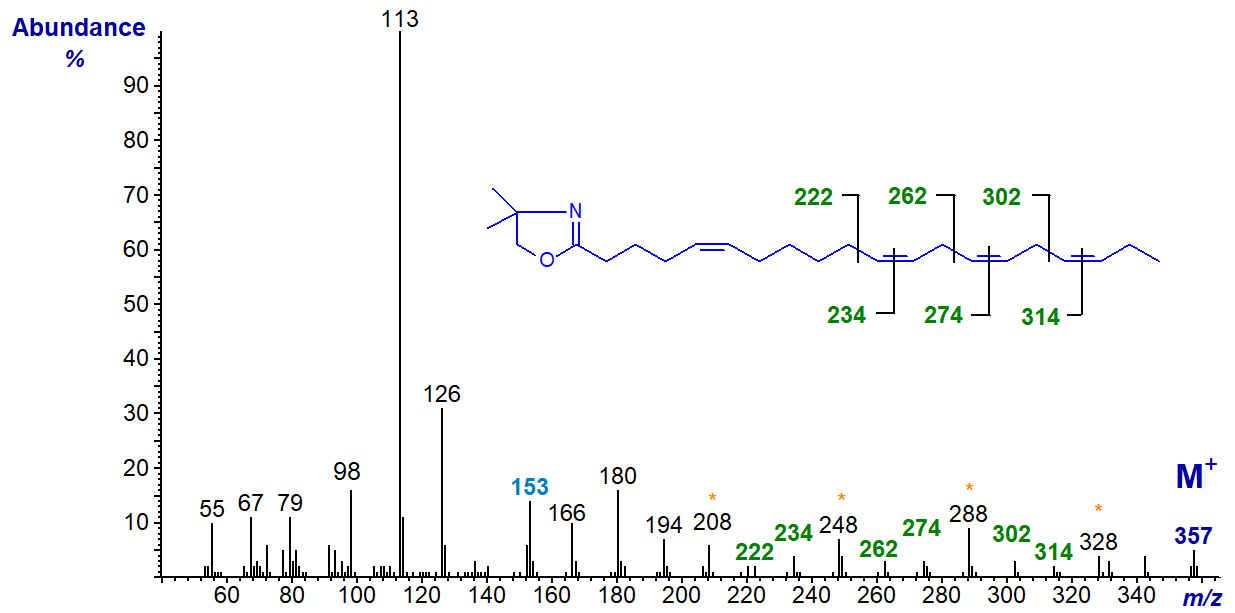
Again, the double bond in position 5 can be deduced from the characteristic fingerprint ion at m/z = 153, together with the abundance of the ion at m/z = 113 relative to that at m/z = 126, while the remaining double bonds are located by the appropriate gaps of 12 or 40 amu, as indicated on the spectrum.
The DMOX derivative of 5,9,19,23-tricosatetraenoate (5,9,19,23-30:4) -
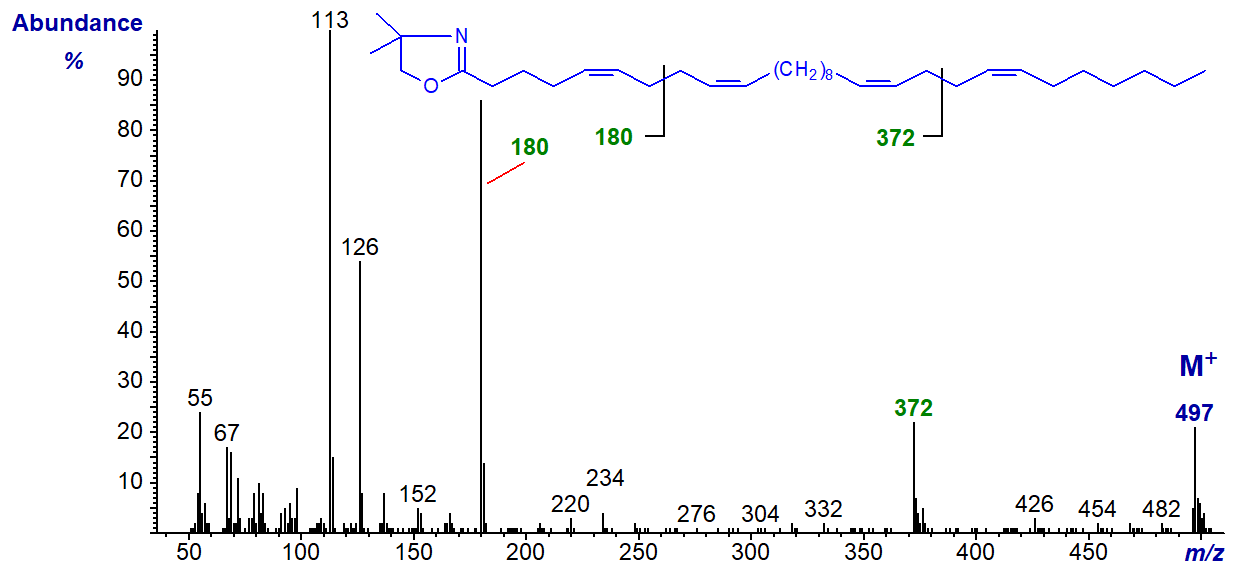
This unusual fatty acid from a sponge has two bis-methylene-interrupted double bond systems, recognized most easily by the characteristic fragmentations at the centre of each, i.e., at m/z = 180 and 372, for the 5,9- and 19,23-double bond systems, respectively.
We have the mass spectrum of the DMOX derivative of 5,9,21,25-32:4 on file in the Archive Section of the web pages, but without interpretation.
Pentaenoic Fatty Acids
Only a few natural fatty acids of this type exist, but they are all of great biological importance. Interpretation of these spectra is the same as for DMOX derivatives of other polyunsaturated fatty acids.
The DMOX derivative of 5,8,11,14,17-eicosapentaenoate (20:5(n-3) or EPA) (Yu, Q.T. et al., 1989; Wolff, R.L. et al., 1999), a common constituent of phospholipids in animals and of fish oils -
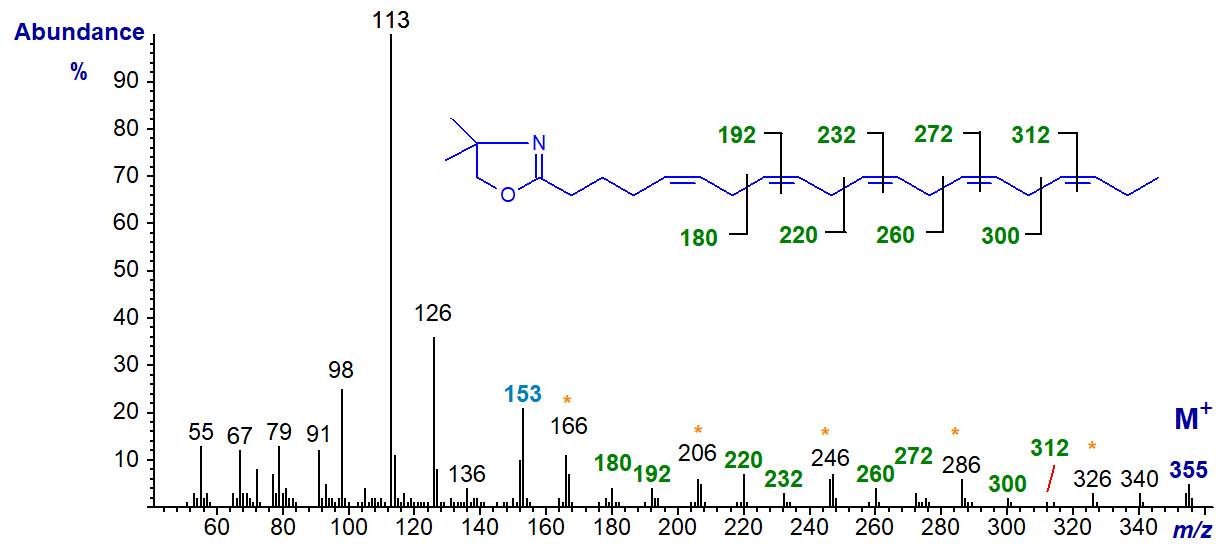
The double bond in position 5 can be deduced from the characteristic fingerprint ion at m/z = 153, while all the remaining double bonds are located by the appropriate gaps of 12 or 40 amu as indicated on the spectrum.
The DMOX derivative of 4,7,10,13,16-docosapentaenoate (22:5(n-6)) (Yu, Q.T. et al., 1989) -
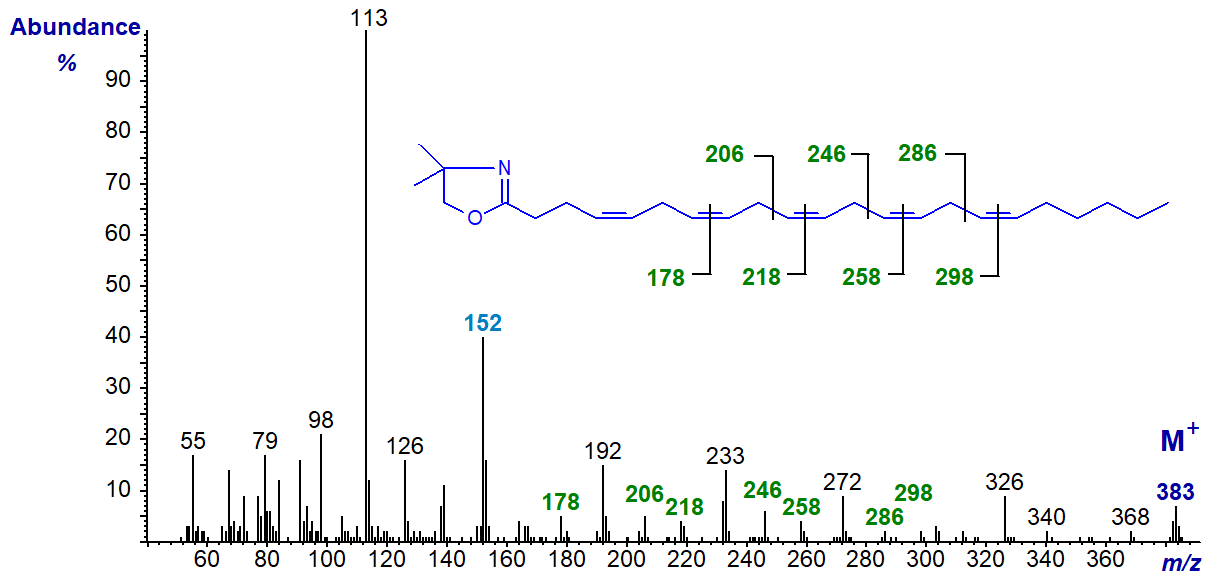
The double bond in position 4 is defined by the fingerprint ion at m/z = 152, but only the later double bonds are clearly delineated otherwise. The relative abundance of the ion at m/z = 113 in comparison to that at m/z = 126 is a further aid to identification of the double bond in position 4.
The DMOX derivative of 7,10,13,16,19-docosapentaenoate (22:5(n-3)) (Yu, Q.T. et al., 1989) -
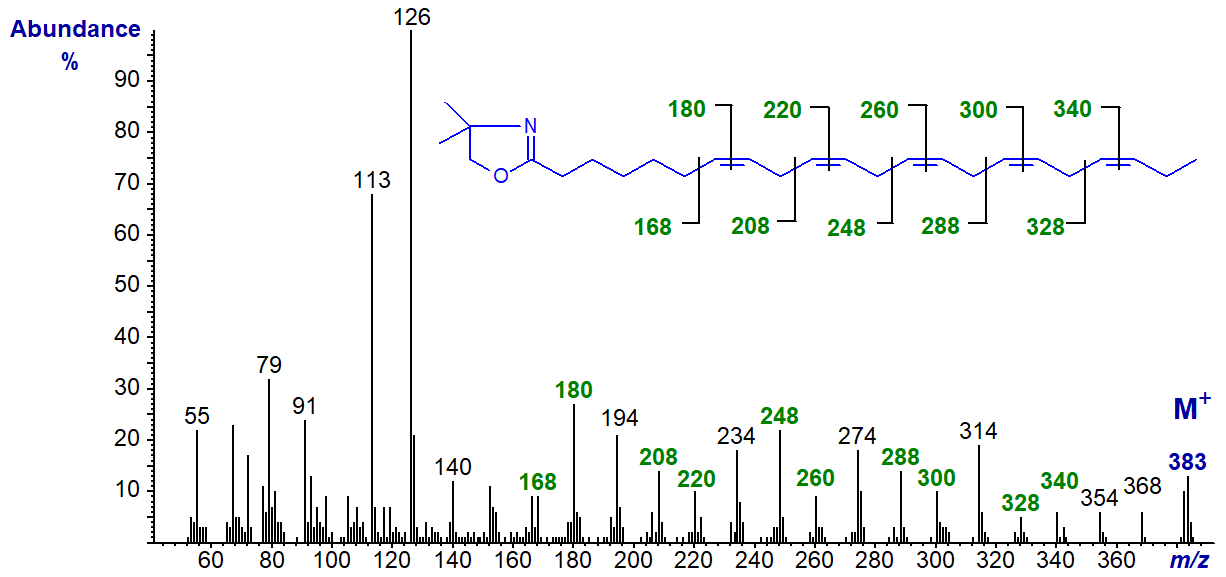
When the first double bond is a little further from the carboxyl group, as in this example, it is not difficult to locate each of the double bonds in the spectrum as indicated. We have the mass spectrum of the DMOX derivative of 6,9,12,15,18-heneicosapentaenoate (21:5(n-3)), a minor but ubiquitous component of fish oils, and of 5,8,11,14,17-octadecapentaenoate (18:5(n‑1)) on file in the Archive Section of the web pages, but without interpretation.
Hexaenoic Fatty Acids
4,7,10,13,16,19-Docosahexaenoate (22:6(n-3) or 'DHA') is a vital component of animal cell membranes, especially those in brain, retina and nervous tissue, and of fish oils. The mass spectrum of its DMOX derivative (Yu, Q.T. et al., 1989; Zhang, J.Y. et al., 1988) is -
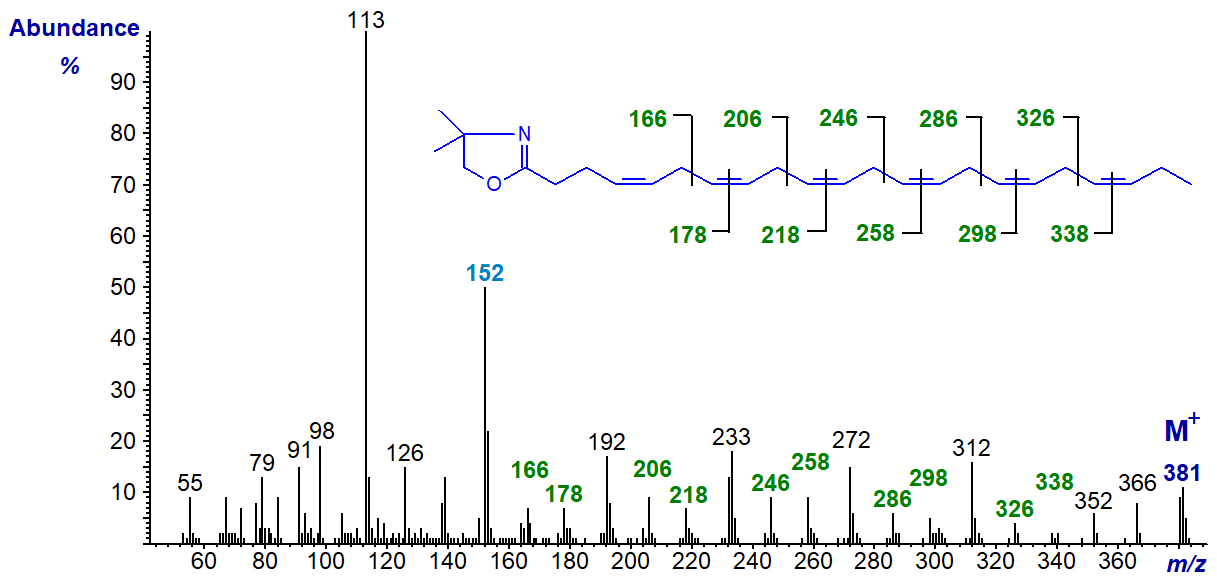
The double bond in position 4 is defined by the fingerprint ion at m/z = 152, and the subsequent double bonds are located by the appropriate gaps of 12 amu, or those of 40 amu between analogous ions.
6,9,12,15,18,21-Tetracosahexaenoate (24:6(n-3)) is a biosynthetic precursor of the previous fatty acid, but it is only occasionally encountered in tissues in significant amounts (in this instance from a jelly fish). The mass spectrum of its DMOX derivative (Nichols, P.D. et al., 2003) is -
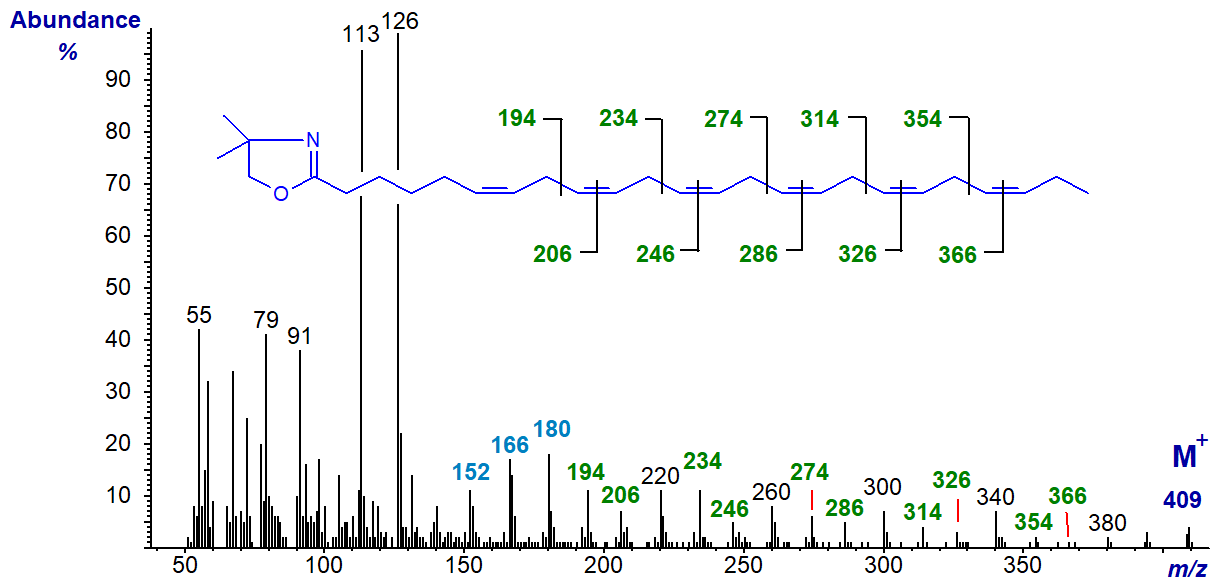
The double bond in position 6 is recognized by the characteristic fingerprint of ions at m/z = 152, 167 and 180, while the remainder are located by the gaps of 12 amu, or those of 40 amu between analogous ions. The ion at m/z = 126 is now more abundant than that at m/z = 113.
In all of these spectra, the tropylium ion at m/z = 91 is small but significant, whereas with the corresponding methyl ester derivatives this can be a dominant ion (see the web page on mass spectra of methyl esters of tetraenoic acids). Presumably this reflects the more orderly fragmentation that occurs with nitrogen-containing derivatives.
References
 Berdeaux, O.
and Wolff, R.L. Gas-liquid chromatography-mass spectrometry of the 4,4-dimethyloxazoline derivatives
of Δ5-unsaturated polymethylene-interrupted fatty acids from conifer seed oils. J. Am. Oil Chem. Soc., 73,
1323-1326 (1996); DOI.
Berdeaux, O.
and Wolff, R.L. Gas-liquid chromatography-mass spectrometry of the 4,4-dimethyloxazoline derivatives
of Δ5-unsaturated polymethylene-interrupted fatty acids from conifer seed oils. J. Am. Oil Chem. Soc., 73,
1323-1326 (1996); DOI.- Hennessy, A.A., Barrett, E., Ross, R.P., Fitzgerald, G.F., Devery, R. and Stanton, C. The production of conjugated α-linolenic, γ-linolenic and stearidonic acids by strains of Bifidobacteria and Propionibacteria. Lipids, 47, 313-327 (2012); DOI.
- Luthria, D.L. and Sprecher, H. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for the structural elucidation of isomeric unsaturated fatty acids. Lipids, 28, 561-564 (1993); DOI.
- Nichols, P.D., Danaher, K.T. and Koslow, J.A. Occurrence of high levels of tetracosahexaenoic acid in the jellyfish Aurelia sp. Lipids, 38, 1207-1210 (2003); DOI.
- Sayanova, O., Smith, M.A., Lapinskas, P., Stobart, A.K., Dobson, G., Christie, W.W. and Shewry, P.R. Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc. Natl. Acad. Sci. USA, 94, 4211-4216 (1997); DOI.
- Wolff, R.L., Christie, W.W., Pedrono, F. and Marpeau, A.M. Arachidonic, eicosapentaenoic, and biosynthetically related fatty acids in the seed lipids from a primitive gymnosperm, Agathis robusta. Lipids, 34, 1083-1097 (1999); DOI.
- Yu, Q.T., Liu, B.N., Zhang, J.Y. and Huang, Z.H. Location of double bonds in fatty acids of fish oil and rat testis lipids. Gas chromatography-mass spectrometry of the oxazoline derivatives. Lipids, 24, 79-83 (1989); DOI.
- Zhang, J.Y., Yu, Q.T., Liu, B.N. and Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom., 15, 33-44 (1988); DOI.
| © Author: William W. Christie |  |
|
| Updated: November 8th, 2023 | Contact/credits/disclaimer | |
