Mass Spectrometry of DMOX Derivatives
Trienoic Fatty Acids
Methylene-Interrupted Trienoic Fatty Acids
The mass spectra of DMOX derivatives of trienoic fatty acids permit location of the double bonds but less easily than with those of monoenes and dienes. The principles described in the earlier documents in this series apply, so we are in general looking for the diagnostic gaps of 12 amu between significant ions. When the first double bond is close to the carboxyl group, the general fragmentation rule for double bond location is no longer appropriate and comparison with the 'fingerprint' spectrum for the relevant monoene derivative is an invaluable guide to its identification.
While identification from first principles can sometimes be problematic, different isomers always seem to have very different spectra so that characterization is possible when spectra of DMOX derivatives of authentic fatty acids can be compared. Unlike the dienes, few model compounds are available for study, so most of the following spectra have been gleaned from analyses of natural products in my own laboratory, not from a systematic study, and fatty acids with a variety of chain-lengths are described. References are listed when we are aware of prior formal publication of spectra in the scientific literature.
The mass spectrum of the DMOX derivative of 6,9,12-octadecatrienoate (γ-linolenate or 18:3(n-6)) is illustrated below (Sayanova, O. et al., 1997) -
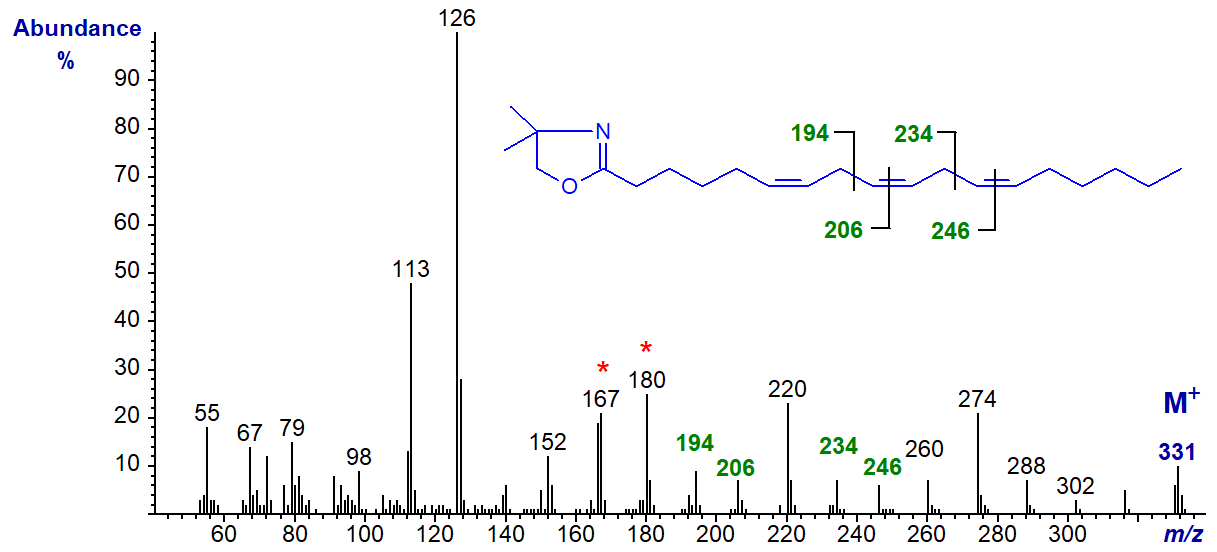
The double bonds in positions 9 and 12 are easily recognized from the gaps of 12 amu between m/z = 194 and 206 and between 234 and 246, respectively. That in position 6 must be identified by the fingerprint characteristic for an isomer with the first double bond in position 6, i.e., the odd-numbered ion at m/z = 167 (or the triplet at m/z = 167, 180 and 194).
The DMOX derivative of 8,11,14-octadecatrienoate (18:3(n-4)) - a minor component of fish oils -
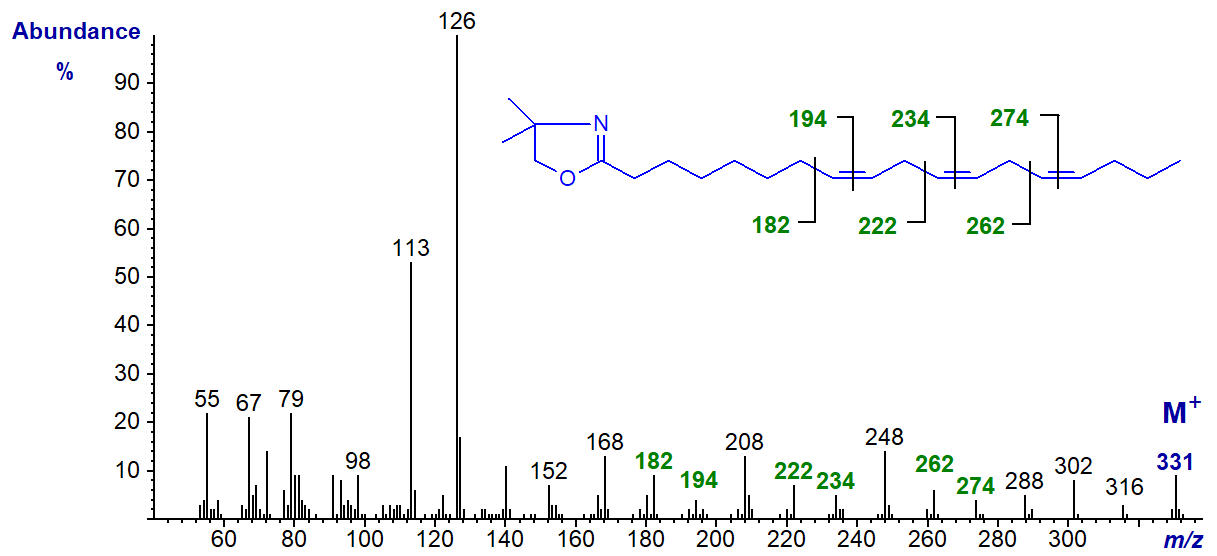
The DMOX derivative of the ubiquitous 9,12,15-octadecatrienoate (α-linolenate or 18:3(n-3)) (Zhang et al., 1988) -
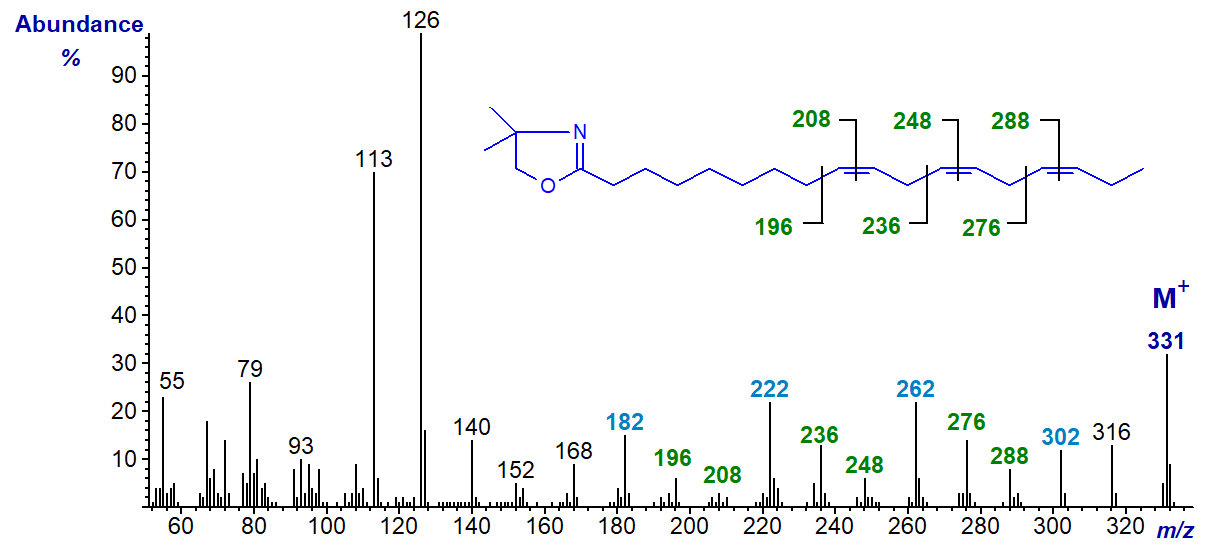
In this and the previous example, all three double bonds are easily recognized by the gaps of 12 amu as indicated in the spectra. Thus, in the last, gaps of 12 amu between m/z = 196 and 208, 236 and 248, and 276 and 288 locate the double bonds in position 9, 12 and 15, respectively. Note that with methylene-interrupted double bonds, the gaps of 40 amu between m/z = 196, 236 and 276, between m/z = 208, 248 and 288, and especially between m/z = 182, 222 and 302 are useful diagnostically as indicated on the spectrum. Comparable ions but 14 amu less are present in the spectrum of the 8,11,14-isomer illustrated above.
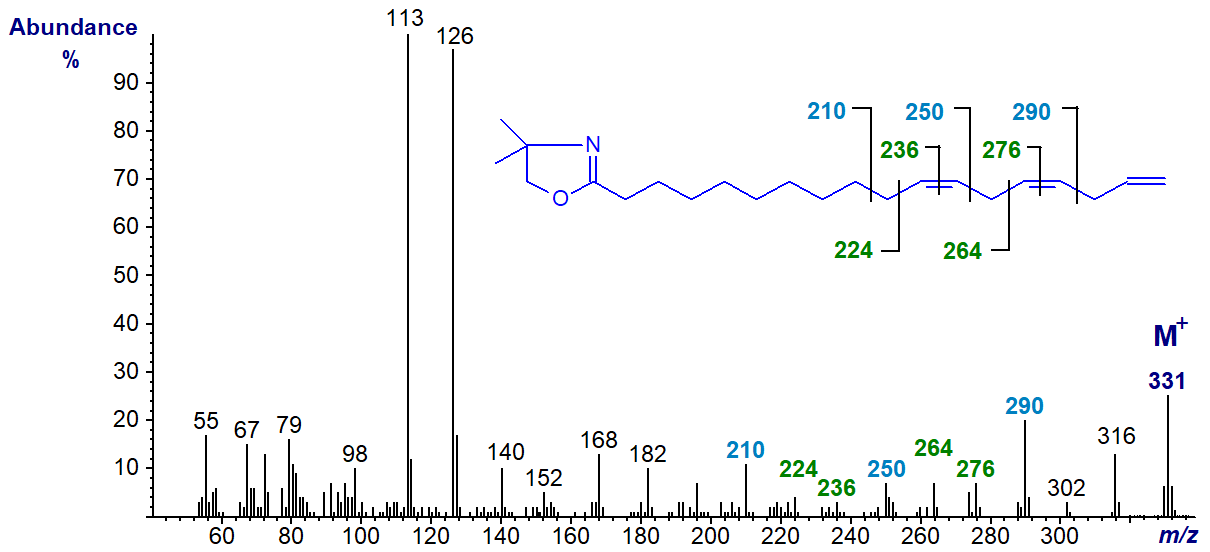
Trienes of the n-1 family of fatty acids have yet to be found in nature, but this spectrum (from a genetically modified plant) is illustrated next for the sake of completeness (Sayanova, O. et al., 2006). The first two double bonds are easily located but terminal double bonds give problems with all derivative types, but especially because of the ease with which a methyl group is lost from the heterocyclic ring in DMOX derivatives. Here again the gaps of 40/41 amu are useful indicators (m/z = 210 to 250 to 290 to 331). The DMOX derivative of 11,14,17-octadecatrienoate (18:3(n-1)) -
There are three important eicosatrienoic acids in animal system, the first of which illustrated here is found during essential fatty acid deficiency and is often termed Mead's acid; the DMOX derivative of 5,8,11-eicosatrienoate (18:3(n-9)) is -
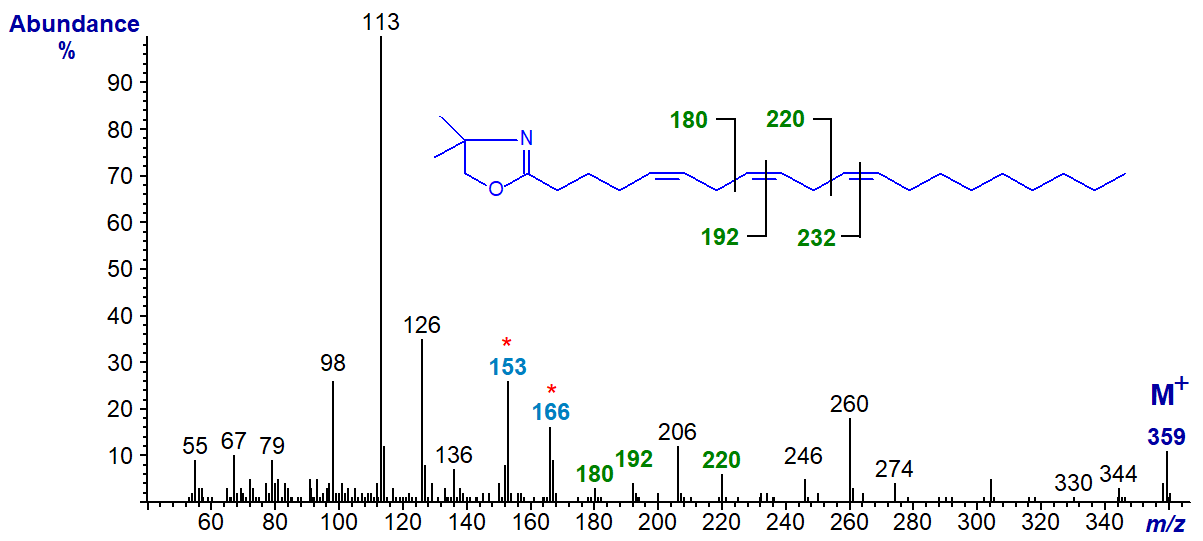
The double bond in position 5 must be recognized from the fingerprint ions at m/z = 153 and 166, but the remaining ions are located as marked. All three double bonds are easily recognized by the gaps of 12 amu, as indicated, in the spectra of the two 20:3 isomers that follow. Thus, the DMOX derivative of 8,11,14-eicosatrienoate (20:3(n-6)) -
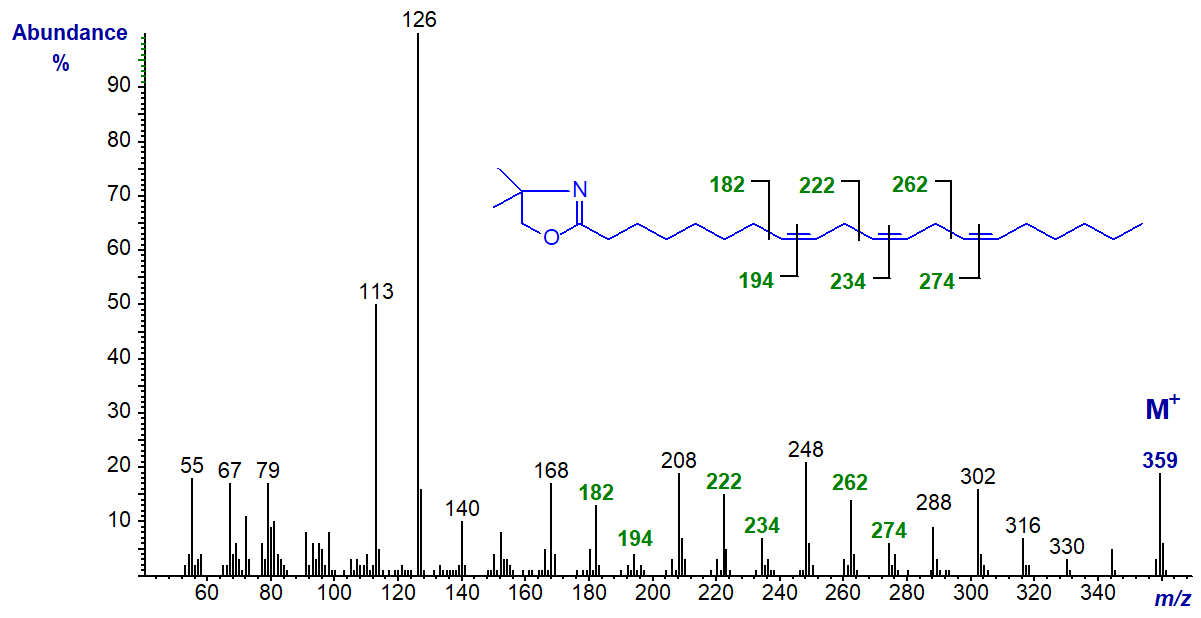
The DMOX of 11,14,17-eicosatrienoate (11,14,17-20:3 or 20:3(n-3)) (Wallis and Browse, 1999) -
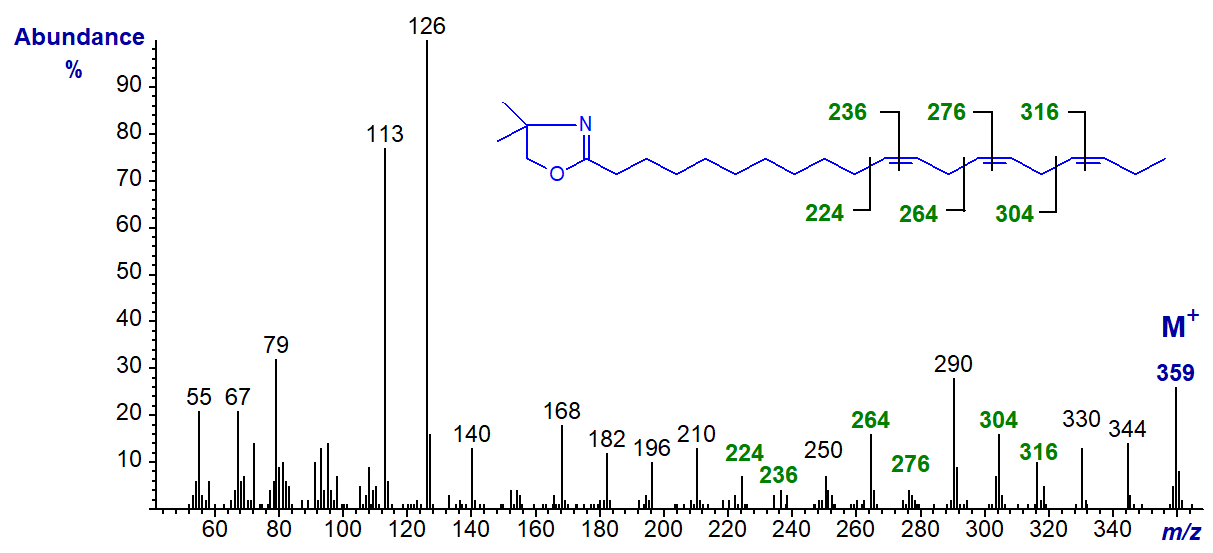
We have the spectra of the DMOX derivatives of further methylene-interrupted trienes in our Archive, including four 16:3 isomers, only one of which is illustrated below, i.e., the DMOX derivative of 4,7,10-hexadecatrienoate (16:3(n-6)) -
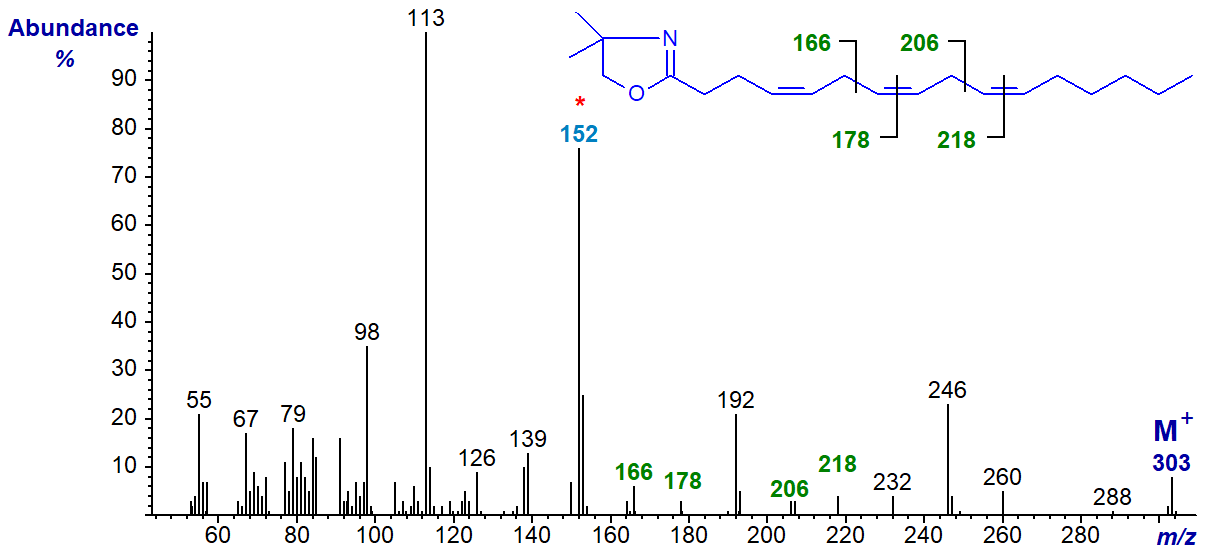
The double bond in position 4 must be recognized from the fingerprint ion at m/z = 152, as in the spectrum of the relevant monoene, but the remaining ions are located as marked.
Trienoic Fatty Acids with Conjugated Double Bonds
Trienoic fatty acids with only two of the double bonds in conjugation are not often encountered in nature, but 9-cis,11-trans,15-cis-octadecatrienoic acid is formed by biohydrogenation of α-linolenic acid in the rumen of cows and sheep and occurs as a minor component of milk fat, adipose tissue and bile of these species. The mass spectrum of its DMOX derivative is -
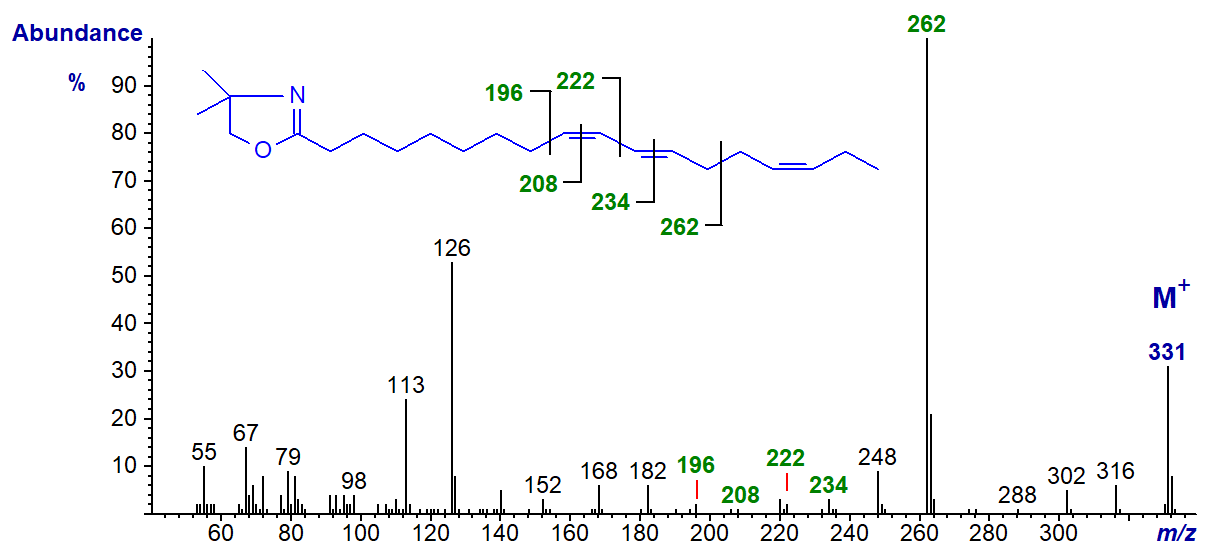
The gap of 12 amu between m/z = 196 and 208 confirms the double bond in position 9, while that for the similar gap between 222 and 234 confirms the double bond in position 11. The outstanding feature of the spectrum is the base ion at m/z = 262, which represents cleavage at the centre of the bis-methylene-interrupted double bond system (see our web page on dienes and below). The gap to m/z = 288 confirms the double bond in position 15. The mass spectrum does not of course give information on the configurations of the double bonds (Destaillats, F. et al., 2005). In the mass spectrum of the DMOX derivative of cis-6,trans-8,cis-12-octadecatrienoate (illustrated here...), the key diagnostic ion is shifted down to m/z = 220 as might be expected (author, not published previously)
Fully conjugated trienoic acids are well known constituents of certain seed oils and the mass spectrum of the DMOX derivative of punicic or 9-cis,11-trans,13‑cis-octadecatrienoic acid follows.
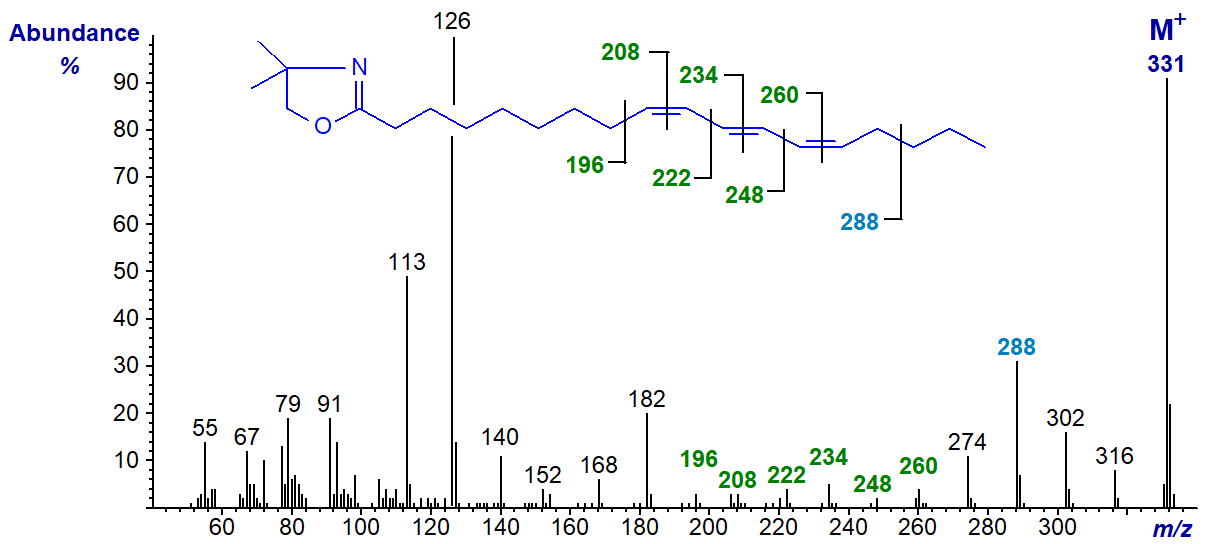
In this instance, all the positions of all the double bonds can be recognized from the gaps of 12 amu as illustrated. The mass spectrum of the DMOX derivative of the geometrical isomer α-eleostearate (9c,11t,13t-18:3) is identical to this and has been published by Spitzer (1997). It is noteworthy that the ion formed by cleavage beta to the double bond system at m/z = 288 is highly abundant. Similarly, an analogous ion at m/z = 246 is especially distinctive in the spectrum of the DMOX derivative of 6,8,10-octadecatrienoate (geometry uncertain), as illustrated in the Archive pages here... (author, not published previously). The tropylium ion at m/z = 91 is a small but significant component of the spectrum only with the last spectrum of a conjugated triene, whereas with methyl ester derivatives of fatty acids with three or more double bonds this can be a dominant ion (see the web page on mass spectra of methyl esters of tetraenoic acids, etc).
There seems little doubt that DMOX derivatives are better than 3-pyridylcarbinol esters and pyrrolidides for locating double bonds in conjugated fatty acids. On the other hand, there is a danger of isomerization occurring if the derivatization conditions are too vigorous.
Trienoic Fatty Acids with Bis- and Polymethylene-Interrupted Double Bonds
As described elsewhere for dienes, it has become apparent that bis- and polymethylene-interrupted trienoic fatty acids are more common in nature than may have been supposed, and fatty acids with a 5,9-double bond system or their chain elongation products are present in seed oils from Gymnosperms or in certain marine invertebrates such as sponges.
The spectrum of the DMOX derivative of 5,9,12-octadecatrienoate (pinolenic acid) from a pine species is typical (Fay and Richli, 1991).
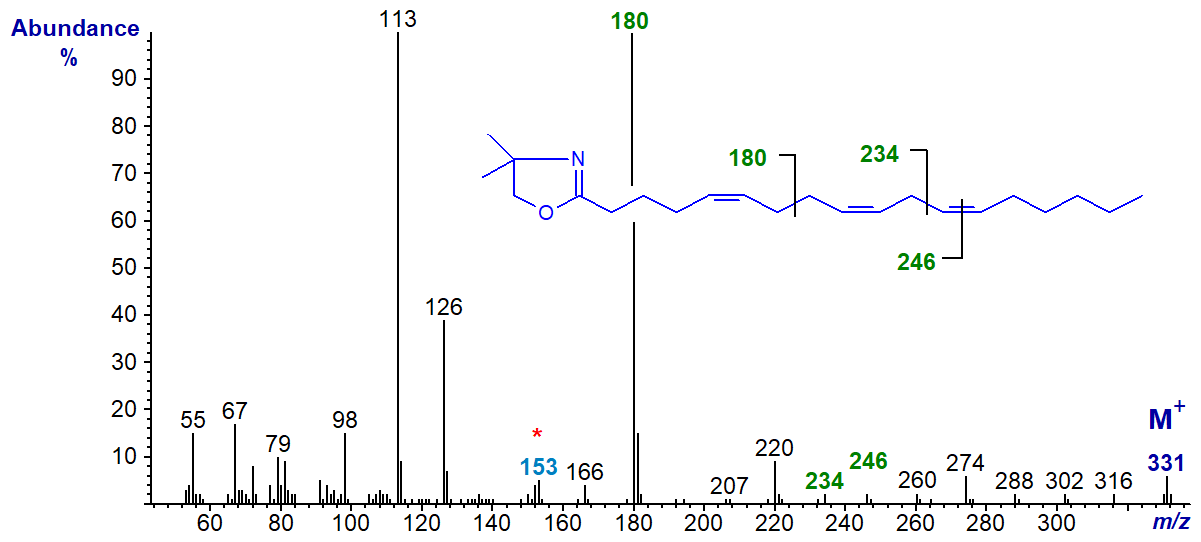
As described for (bis-methylene-interrupted dienes, the prominent ion at m/z = 180 represents cleavage at the centre of the bis-methylene-interrupted (5,9) double bond system. Analogous ions are present in the corresponding spectra of pyrrolidides and 3‑pyridylcarbinol ('picolinyl') esters. The double bond in position 12 is located by a gap of 12 amu between m/z = 234 and 246. Note that the odd-numbered ion at m/z = 153 is diagnostic for a double bond in position 5, while the fact that the ion at m/z = 113 is so much larger than that at m/z = 126 is also characteristic (see the corresponding web page on monoenes).
The spectrum of the DMOX derivative of 5,9,13-eicosatrienoate (5,9,13-20:3) from a sponge appears to be the only one of its kind to have been discovered so far in that it has two adjacent bis-methylene-interrupted double bond systems -
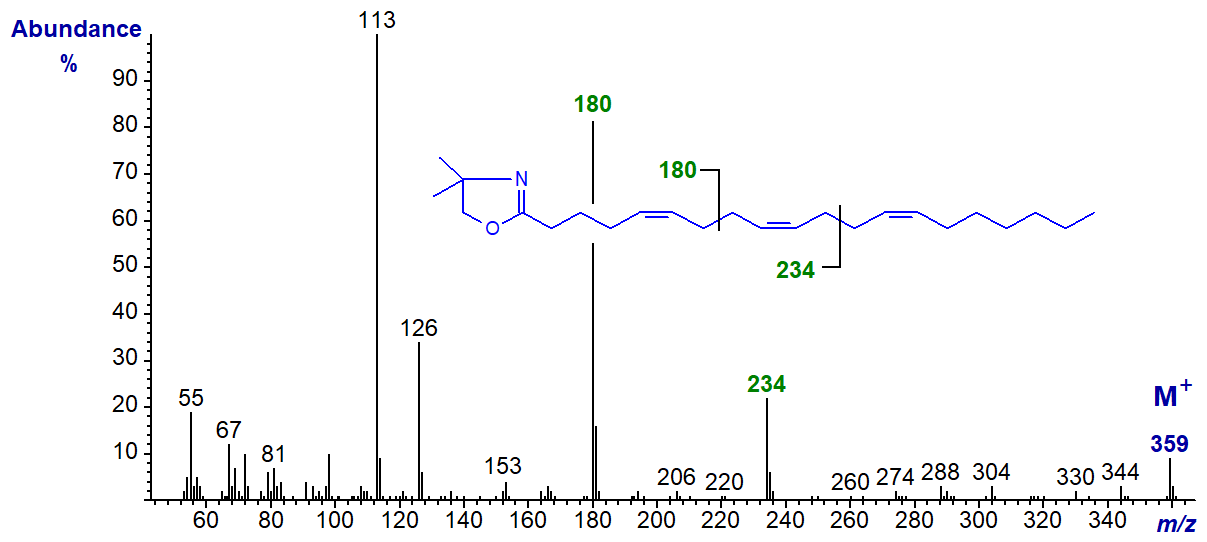
The ion at m/z = 180 represents cleavage at the centre of the 5,9-double bond system, while that at m/z = 234 is diagnostic for the 9,13‑system. Although ions for the individual double bonds are not easily recognized, this hardly matters with such a unique spectrum.
The DMOX derivative of 5,9,19-hexacosatrienoate (5,9,19-26:3) -
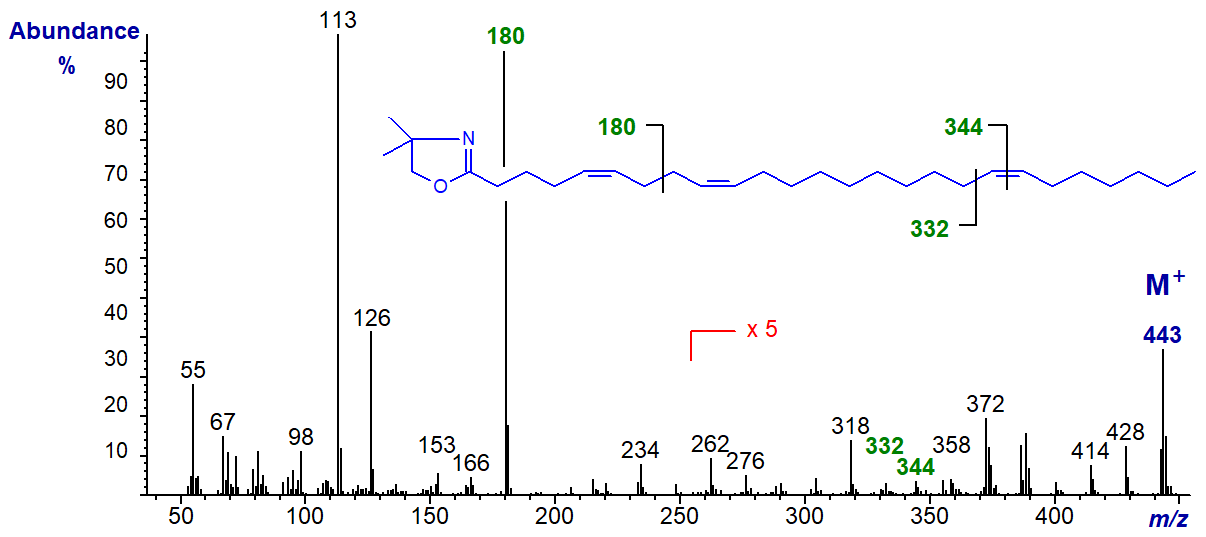
This very-long chain triene is typical of the type of fatty acid component found in sponges, in this instance from Hymeniacidon cinerea. It has the ion at m/z = 180 for the 5,9‑double bond system, while the gap of 12 amu later as indicated on the spectrum locates that in position 19.
The spectrum of the DMOX derivative of 7,11,14-eicosatrienoate (7,11,14-20:3) from seed oils of pine species.
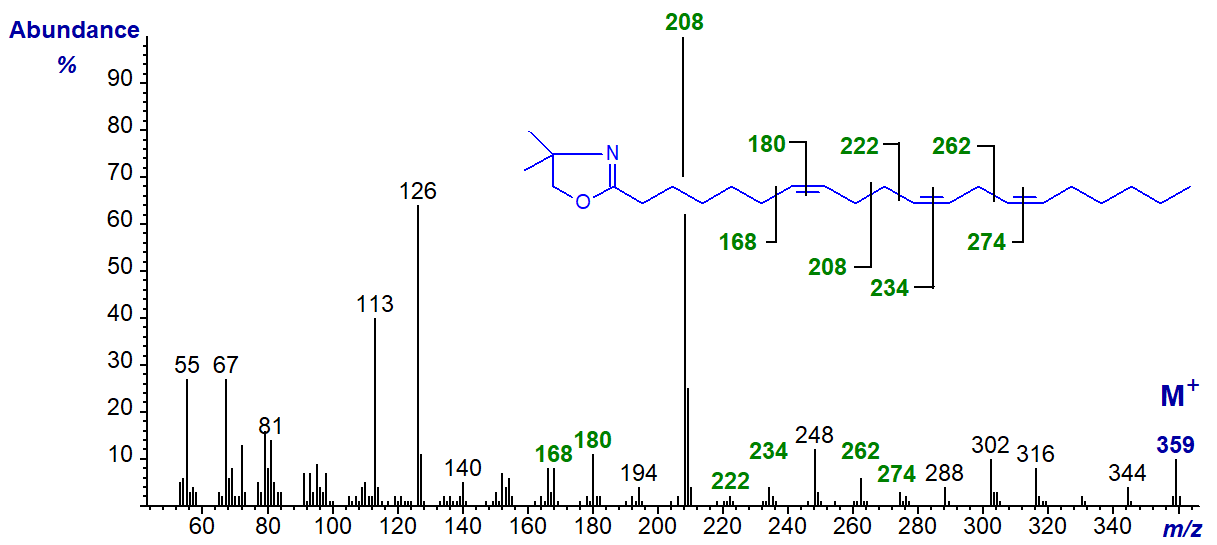
In this instance, the spectrum resembles that of 5,9,12-18:3 except that the diagnostic ions are shifted upwards by 28 amu (Wolff, R.L. et al., 1997)). In particular, the ion representing cleavage at the centre of the bis-methylene-interrupted double bond system is now at m/z = 208.
A few trienoic fatty acids are known in which double bonds are separated by more than two methylene groups as in certain conifer species. The spectrum of the DMOX derivative 5,11,14-eicosatrienoate (5,11,14-20:3 or sciadonic) from Pinus contorta seed oil follows (see Zhang, J.Y. et al., 1988) -
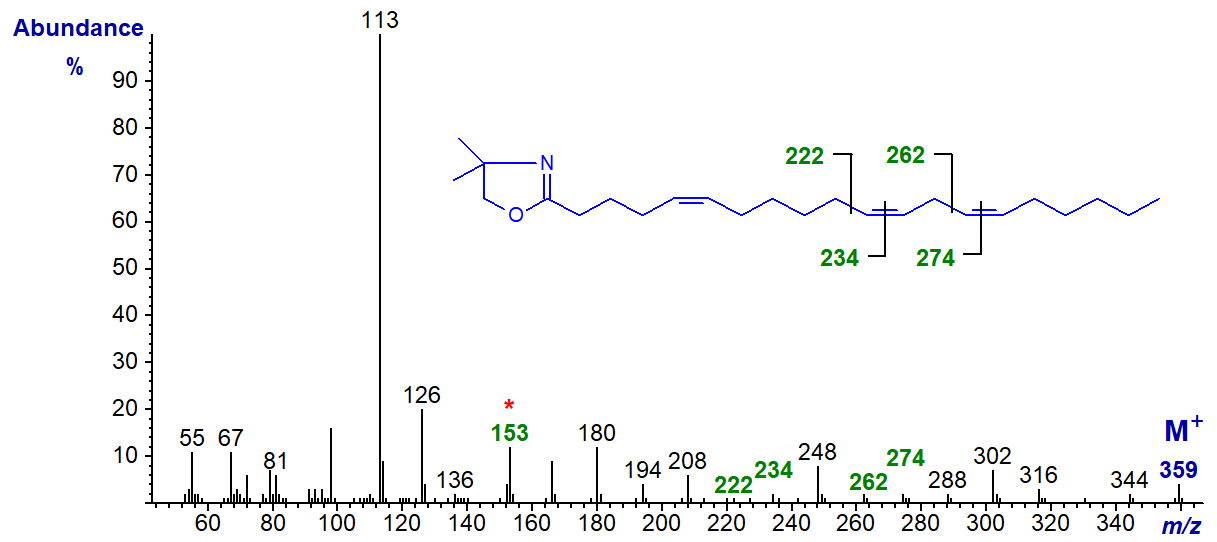
All the double bonds must be identified individually - that in position 5 by the diagnostic ion at m/z = 153 and the others by the gaps of 12 amu.
A few fatty acids with isolated double bonds in position 3 are found in plants, although these are often of the trans rather than the cis configuration as in the example here, i.e., the DMOX derivative of 3,9,12-octadecatrienoate from Tanacetum zawadskii seed oil (Tsevegsuren, N. et al., 2003).
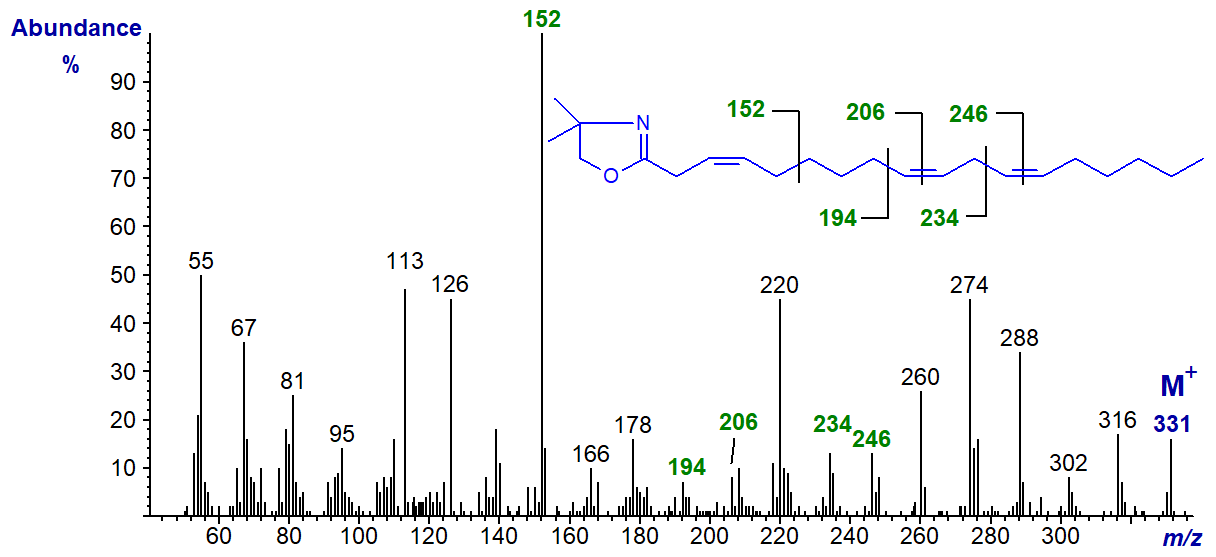
The base ion at m/z = 152 is the important diagnostic feature, though this would also be the case if the double bond were in position 2, and it is possible that isomerization has occurred during derivatization (see our web page on DMOX derivatives of monoenes).
We have mass spectra of the DMOX derivatives of further trienoic fatty acids on file and these are illustrated in the Archive Section of these web pages but without detailed interpretation. Most of these have not been formally published elsewhere.
References
 Destaillats, F.,
Trottier, J.P., Galvez, J.M.G. and Angers, P. Analysis of α-linolenic acid biohydrogenation intermediates in milk fat
with emphasis on conjugated linolenic acids. J. Dairy Sci., 88, 3231-3239 (2005);
DOI.
Destaillats, F.,
Trottier, J.P., Galvez, J.M.G. and Angers, P. Analysis of α-linolenic acid biohydrogenation intermediates in milk fat
with emphasis on conjugated linolenic acids. J. Dairy Sci., 88, 3231-3239 (2005);
DOI.- Fay, L. and Richli, U. Location of double bonds in polyunsaturated fatty acids by gas chromatography-mass spectrometry after 4,4-dimethyloxazoline derivatization. J. Chromatogr. A, 541, 89-98 (1991); DOI.
- Sayanova, O., Haslam, R., Guschina, I., Lloyd, D., Christie, W.W., Harwood, J.L. and Napier, J.A. A bifunctional Δ12, Δ15-desaturase from Acanthamoeba castellanii directs the synthesis of highly unusual n-1 series unsaturated fatty acids. J. Biol. Chem., 281, 36533-36541 (2006); DOI.
- Sayanova, O., Smith, M.A., Lapinskas, P., Stobart, A.K., Dobson, G., Christie, W.W. and Shewry, P.R. Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc. Natl. Acad. Sci. USA, 94, 4211-4216 (1997); DOI.
- Spitzer, V. Structure analysis of fatty acids by gas chromatography - low resolution electron impact mass spectrometry of their 4,4-dimethyloxazoline derivatives - a review. Prog. Lipid Res., 35, 387-408 (1997); DOI.
- Tsevegsuren, N., Fujimoto, K., Christie, W.W. and Endo, Y. Occurrence of a novel cis,cis,cis-octadeca-3,9,12-trienoic (Z,Z,Z-octadeca-3,9,12-trienoic) acid in Chrysanthemum (Tanacetum) zawadskii Herb. (Compositae) seed oil. Lipids, 38, 573-578 (2003); DOI.
- Wallis, J.G. and Browse, J. The Δ8-desaturase of Euglena gracilis: An alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch. Biochem. Biophys., 365, 307-316 (1999); DOI.
- Wolff, R.L., Christie, W.W. and Coakley, D. Bishomopinolenic (7,11,14-20:3) acid in Pinaceae seed oils. J. Am. Oil Chem. Soc., 74, 1583-1586 (1997); DOI.
- Zhang, J.Y., Yu, Q.T., Liu, B.N. and Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom., 15, 33-44 (1988); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
