Mass Spectrometry of Fatty Acid Pyrrolidides
Dienoic fatty acids. Part 2. Conjugated and
Bis- and Polymethylene-Interrupted Dienes
In this web page, the electron-impact mass spectra of pyrrolidides are described of those dienoic fatty acids with conjugated double bond systems and others with two or more methylene groups between the double bonds, including those most likely to be encountered in nature together with some useful synthetic fatty acids of this type. Part 1 of this topic describes the spectra of the pyrrolidides of methylene-interrupted dienoic acids.
Interpretation of the mass spectra of pyrrolidides of dienoic fatty acids is similar to that discussed in some detail in the web page here dealing with monoenes, i.e., it is necessary to look for the gap of 12 amu that locates each double bond. As it is not always easy to locate the first double bond especially, the 'fingerprint' of the appropriate monoene is a useful guide. As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum. Interpretation can sometimes be aided by magnifying the ions in the high mass range relative to the base ion. Most of the spectra illustrated here have not been published elsewhere in the scientific literature. The web page on pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). As with other fatty acid pyrrolidides, there are comparable features in the spectra of the corresponding DMOX derivatives.
Conjugated Dienoic Fatty Acids
The mass spectrum of the pyrrolidide of the conjugated diene 9-cis,11-trans-octadecadienoate (a 'CLA' isomer) is illustrated first (Iversen, S.A. et al., 1984) -
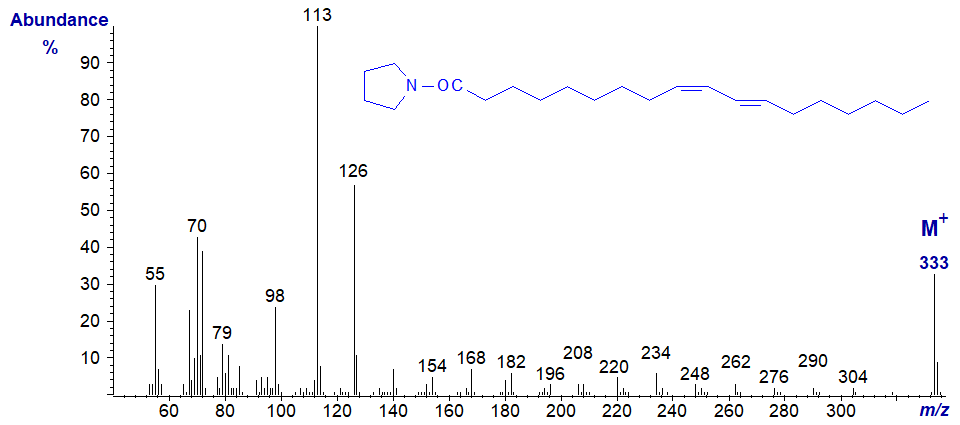
Pyrrolidide of 10-trans,12-cis-octadecadienoate -
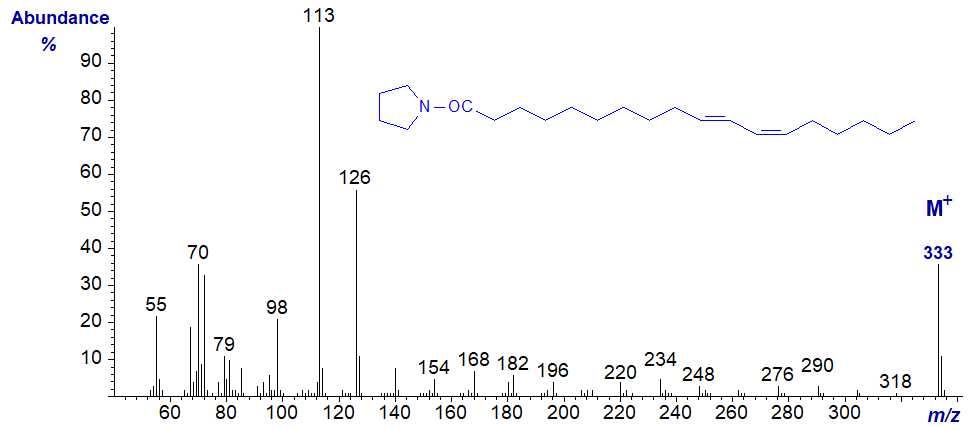
In this instance, the ions in the high mass range have not been magnified, and there is an especially abundant molecular ion, but it is not easy to locate the double bonds with confidence using the established rules as both isomers have very similar mass spectra. DMOX derivatives are certainly much more useful with conjugated fatty acids.
Bis-Methylene-Interrupted Dienoic Fatty Acids
Fatty acids with double bonds in the 5,9-position, i.e., with two methylene groups between the double bonds (or metabolites of these), are common in marine sponges and in plants of the Gymnosperm family. They display rather distinctive fragmentations that serve to locate the double bonds. Many of these spectra have not been published formally elsewhere.
The mass spectrum of the pyrrolidide of 5,9-hexadecadienoate (5,9-16:2) from a sponge (Carballeira, N.M. and Maldonado, L., 1986) -
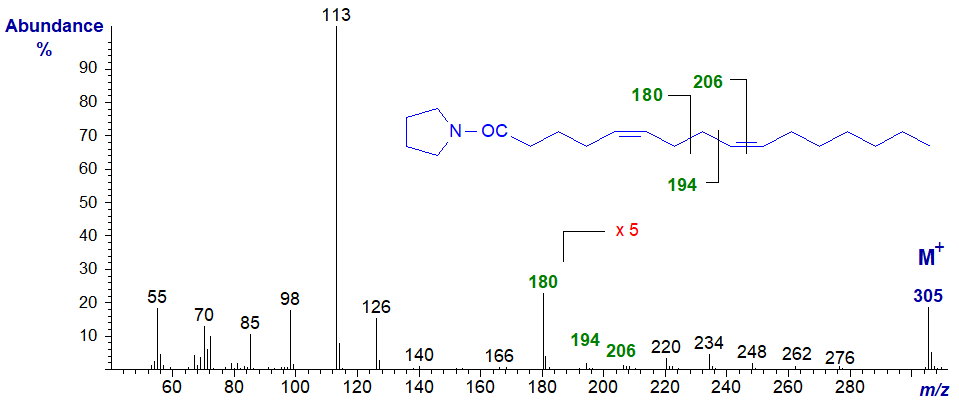
As with all derivatives with a double bond in position 5, the ions in the higher mass range are not very abundant, but a distinctive ion at m/z = 180 is formed by cleavage at the centre of the bis-methylene-interrupted double bond system. An analogous fragmentation is found with all the nitrogen-containing derivatives of fatty acids. The gap of 12 amu between the ions at m/z = 194 and 206 confirms that there is a double bond in position 12.
The mass spectrum of the pyrrolidide of 7,11-octadecadienoate (7,11-18:2) from a sponge -
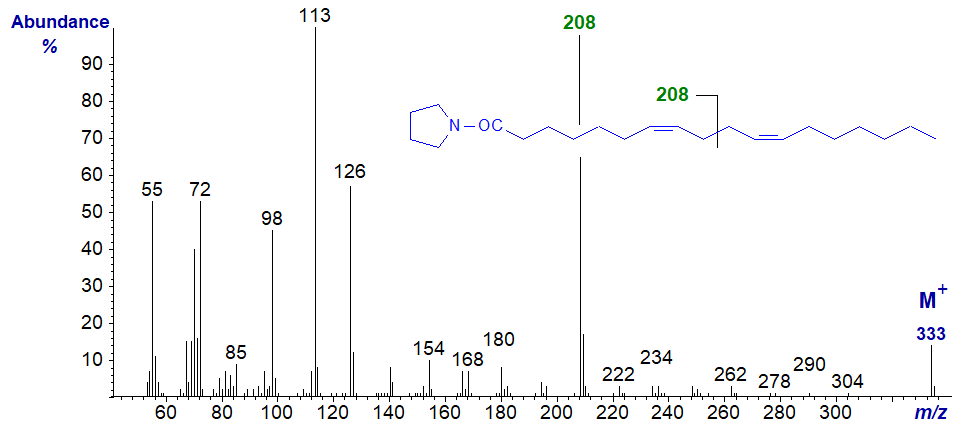
The diagnostic ion for cleavage at the centre of the bis-methylene-interrupted double bond system has now shifted up 28 amu to m/z = 208. It might be easy to confuse the location of the other double bonds by looking for the gaps of 12 amu, but that hardly matters with such a distinctive spectrum.
The mass spectrum of the pyrrolidide of 9,13-eicosadienoate (9,13-20:2) from a sponge -
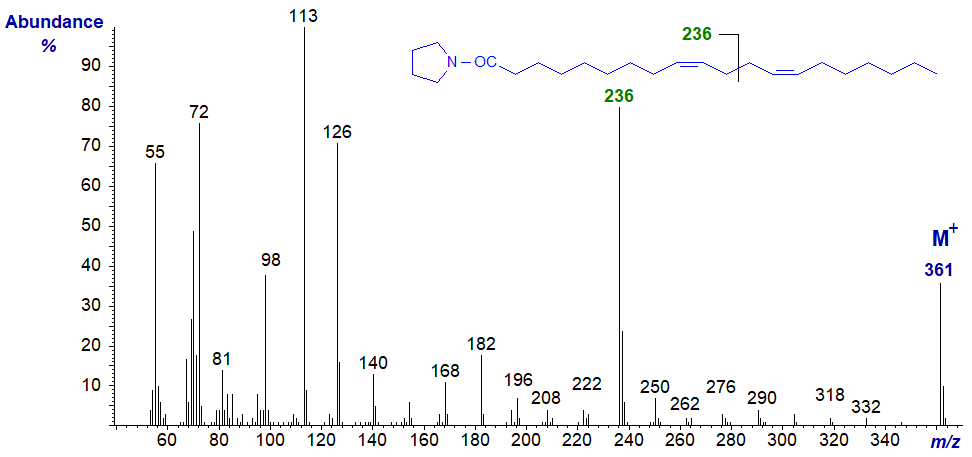
The diagnostic ion for cleavage at the centre of the bis-methylene-interrupted double bonds is now at m/z = 236, with further evidence for location of the double bonds in positions 9 and 13 being provided by gaps of 12 amu between m/z = 196 and 208, and 250 and 262, respectively.
The pyrrolidide of 17,21-octacosadienoate (17,21-28:2) from a sponge -
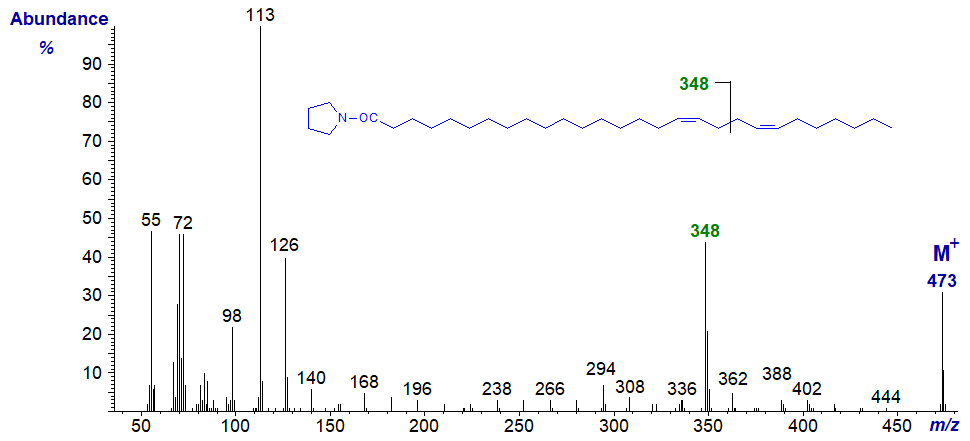
Even with very-long-chain fatty acids of this kind, characterization is possible from the mass spectrum as illustrated, and we can locate additional structural features in such fatty acids, cf., the spectrum of the pyrrolidide of 23-methyl-tetracos-5,9-dienoate illustrated here..., where the methyl branch point is easily located (I leave this to the reader).
When the double bonds are close to the carboxyl group, we still see this distinctive type of fragmentation, as in the mass spectrum of the pyrrolidide of synthetic 2,6‑octadecadienoate (2,6-18:2) -
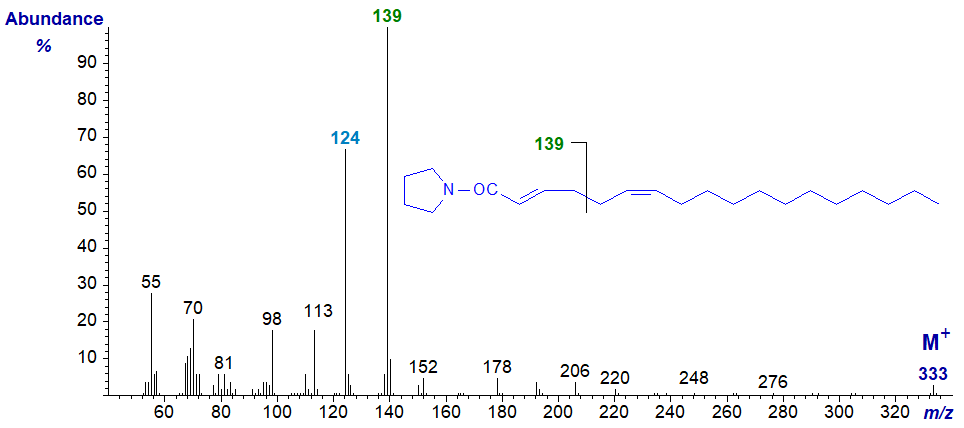
In this instance, the ion at m/z = 139 represents cleavage at the centre of the double bond system, while that at m/z = 124 is characteristic of a double bond in position 2.
Polymethylene-Interrupted Dienoic Fatty Acids
Fatty acids with multiple methylene groups between the double bonds are relatively rare in nature, but they do occur, especially in marine organisms and seed oils from Gymnosperms (conifers). The mass spectrum of the pyrrolidide of 5,13-docosadienoate (5,13-22:2) from meadowfoam oil is -
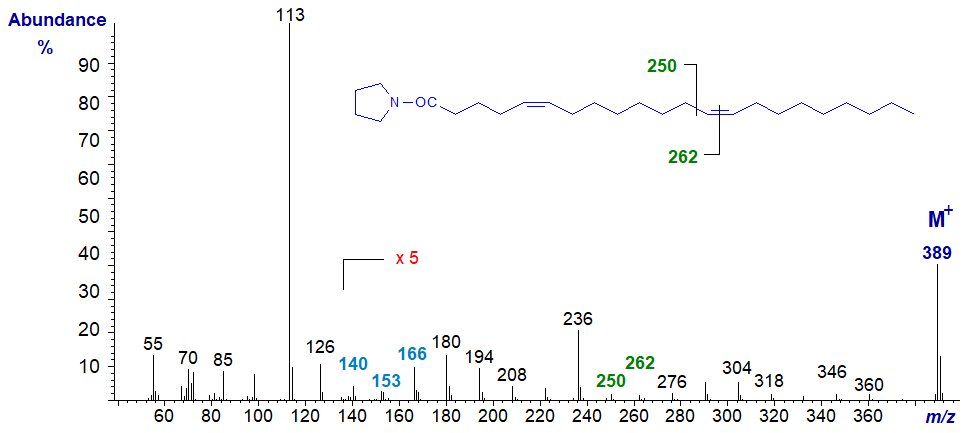
Only by magnifying the ions in the higher mass region can the ions at m/z = 140, 153 and 166 characteristic of a double bond in position 5 be seen (see the web page on monoenes); the fact that magnification is necessary is in itself typical for a 5-double bond. The gap of 12 amu between m/z = 250 and 262 locates the double bond in position 13.
Mass spectrum of the pyrrolidide of 7,15-docosadienoate (7,15-22:2) from a marine invertebrate, Rapana thomasiana, -
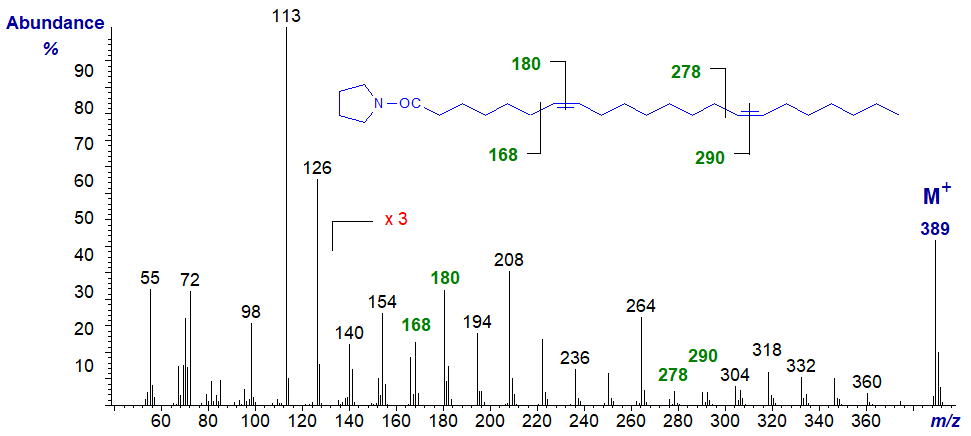
The gaps of 12 amu between m/z = 168 and 180, and between m/z = 278 and 290, locate the double bonds in positions 7 and 15, respectively.
Mass spectra of pyrrolidides of many more dienoic acids of various chain lengths and double bond positions can be found in our Archive Section, but without interpretation. Most of these have not been published elsewhere.
References
- Carballeira, N.M. and Maldonado, L. Identification of 5,9-hexadecadienoic acid in the marine sponge Chondrilla nucula. Lipids, 21, 470-471 (1986); DOI.
- Iversen, S.A., Cawood, P., Madigan, M.J., Lawson, A.M. and Dormandy, T.L. Identification of a diene conjugated component of human lipid as octadeca-9,11-dienoic acid. FEBS Letts, 171, 320-324 (1984); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
