Mass Spectrometry of Fatty Acid Pyrrolidides
Dienoic Fatty Acids. Part 1. Methylene-Interrupted Dienes
 Interpretation
of the mass spectra of pyrrolidides of dienoic fatty acids is similar to that discussed
in some detail in the web page here dealing with pyrrolidides of monoenes,
i.e., it is necessary to look for the gap of 12 amu that locates each double bond.
However, it is not always easy to locate the first double bond especially,
and then the 'fingerprint' spectrum of the appropriate monoene is a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum,
as I have never yet found two positional isomers with identical spectra.
Interpretation can sometimes be aided by magnifying the ions in the high mass range relative to the base ion.
Interpretation
of the mass spectra of pyrrolidides of dienoic fatty acids is similar to that discussed
in some detail in the web page here dealing with pyrrolidides of monoenes,
i.e., it is necessary to look for the gap of 12 amu that locates each double bond.
However, it is not always easy to locate the first double bond especially,
and then the 'fingerprint' spectrum of the appropriate monoene is a useful guide.
As with the other pyrrolidides, it is always helpful to have access to an authentic spectrum,
as I have never yet found two positional isomers with identical spectra.
Interpretation can sometimes be aided by magnifying the ions in the high mass range relative to the base ion.
It is my general impression that pyrrolidides are less distinctive than DMOX or 3‑pyridylcarbinol derivatives when trying to find the key diagnostic ions in an unknown, but they have their own merits. The web page on pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). Part 2 of this topic describes the spectra of the pyrrolidides of most other dienoic acids, including conjugated and bis- and polymethylene-interrupted dienes.
Methylene-Interrupted Octadecadienoates
Fortunately, we had available a series of positional isomers of methylene-interrupted octadecadienoic acids (4,7- to 14,17-18:2), which had been prepared some years ago by total synthesis (Christie and Holman, 1967). Information on the mass spectra of the pyrrolidides of these has been published, together with details of some trienoic acids, but data for most are in tabular form and a few spectra only were illustrated (Andersson, B.A. et al., 1975). Therefore, spectra of many of these dienes are now illustrated below for the first time. The mass spectrum of N-octadec-9,12-dienoylpyrrolidine (linoleate or 9,12‑18:2 or 18:2(n-6)) follows -
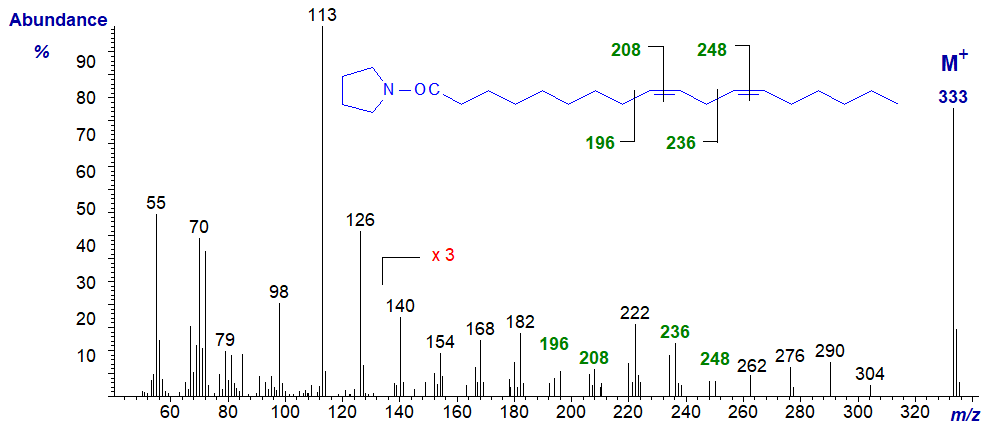
The double bonds in positions 9 and 12 are located by the gaps of 12 amu between ions at m/z = 196 and 208, and 236 and 248, respectively (see our web page on Pyrrolidides of Monoenoic Fatty Acids for details of the rule that applies here). I must confess that if I was faced with this as an unknown it would be difficult to spot these diagnostic ions, and I often find it easier to locate the gaps of 40 amu for fragmentations on either side of the double bonds, i.e., from m/z = 182 to 222 to 262 in this instance. It is always helpful to have an authentic spectrum available.
When the first double bond is relatively close to the carboxyl group, we must compare with the spectrum of the corresponding monoene while considering it simply as a ‘fingerprint’ rather than attempting an interpretation from the fragmentation rule.
Pyrrolidide of 4,7-octadecadienoate (4,7-18:2) -
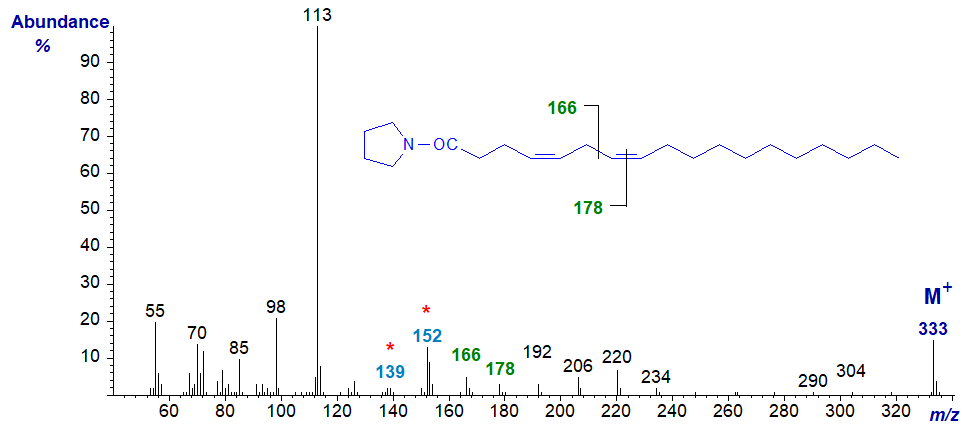
The double bond in position 4 is not readily located from first principles, although the ions at m/z = 139 and 159 are a useful fingerprint for this position. That in position 7 is detected by the gap of 12 amu between m/z = 166 and 178. As the following ions are all 14 amu apart, the first double bond must be in positions 3 to 5 at least.
Pyrrolidide of 5,8-octadecadienoate (5,8-18:2 or sebaleate) -
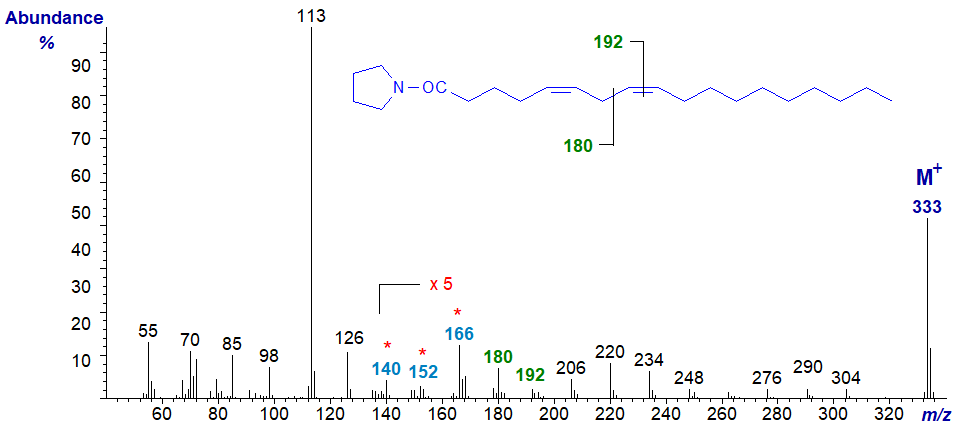
The double bond in position 5 is located by the weak fingerprint ions at m/z = 140 to 166, while a gap of 12 amu (barely discernible) between m/z = 180 and 192 locates the double bond in position 8 (or the gap of 40 between m/z = 166 and 206). Again, all the ions in the higher mass range are 14 amu apart, confirming that there are no double bonds beyond carbon-9 in the molecule.
Pyrrolidide of 6,9-octadecadienoate (6,9-18:2) -
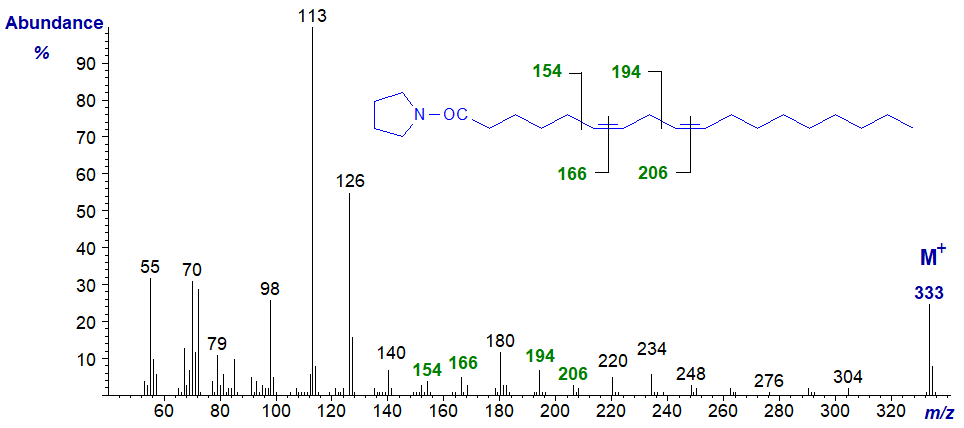
The double bonds in positions 6 and 9 are located by the gaps of 12 amu between m/z = 154 and 166, and 194 and 206, respectively, or the gaps of 40 amu from m/z = 140 to 180 to 220)
Pyrrolidide of 7,10-octadecadienoate (7,10-18:2) -
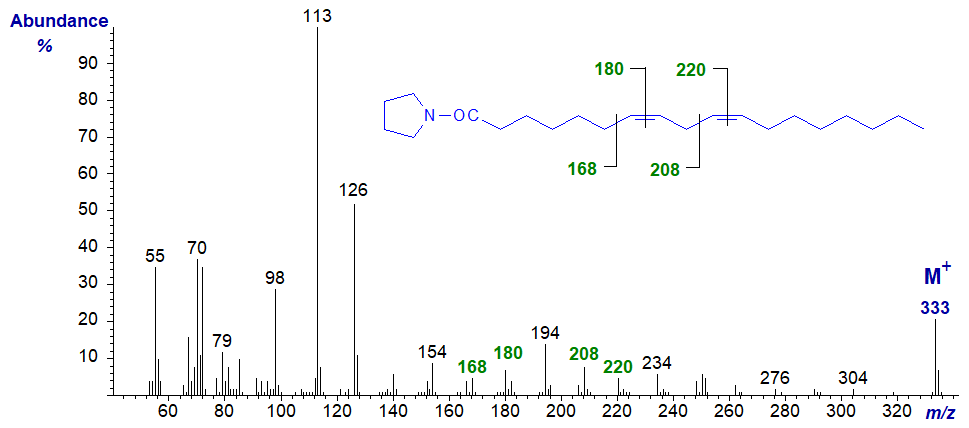
The double bonds in positions 7 and 10 are located by the gaps of 12 amu between m/z = 168 and 180, and 208 and 220, respectively, but I will leave my readers to locate the diagnostic ions for the gap of 40 amu for this and the next few spectra.
Pyrrolidide of 8,11-octadecadienoate (8,11-18:2) -
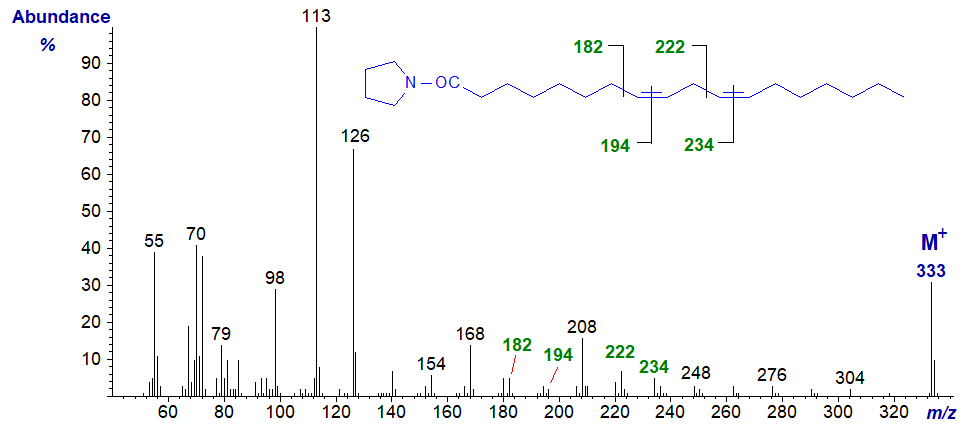
The double bonds in positions 8 and 11 are located by the gaps of 12 amu between m/z = 182 and 194, and 222 and 234, respectively.
Pyrrolidide of 10,13-octadecadienoate (10,13-18:2) -
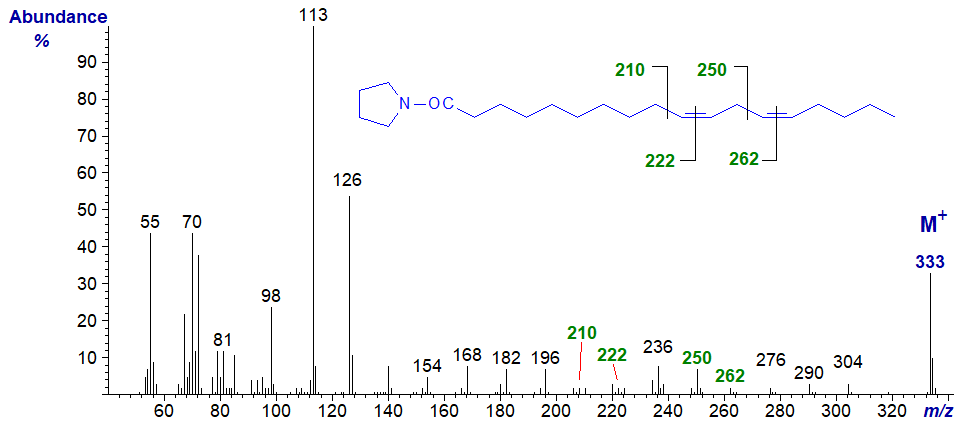
The double bonds in positions 10 and 13 are located by the gaps of 12 amu between m/z = 210 and 222, and 250 and 262, respectively.
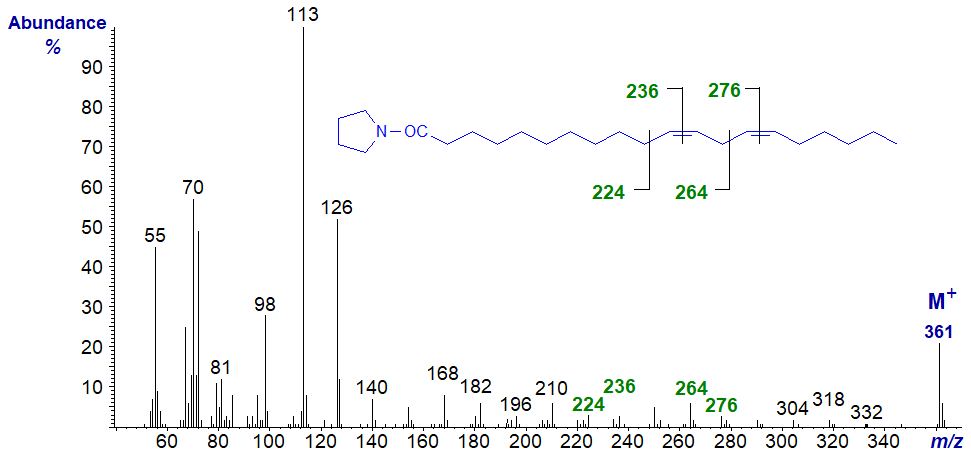
Pyrrolidide of 11,14-octadecadienoate (11,14-18:2) -
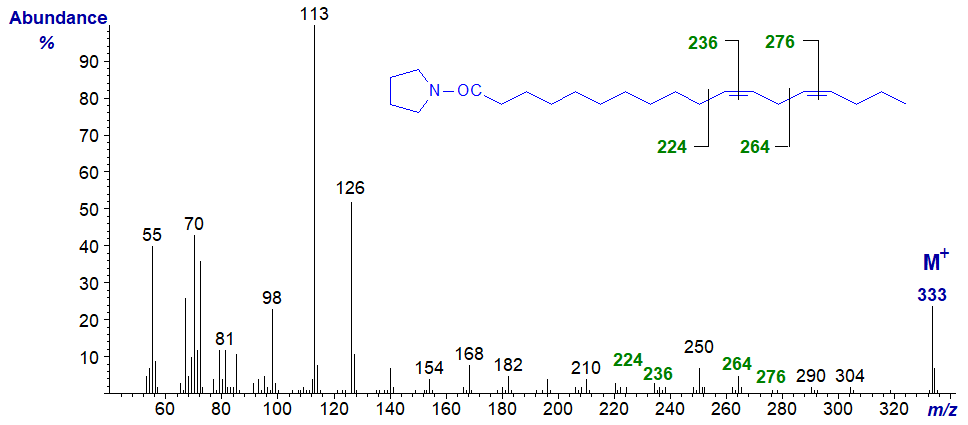
The double bonds in positions 11 and 14 are located by the gaps of 12 amu between m/z = 224 and 236, and 264 and 272, respectively, but they would not be easy to detect from first principles. Here it is again useful to have the authentic spectrum available. At least, it is easy to determine that there are no double bonds before carbon-12 from the regular series of ions 14 amu apart. From now on it is perhaps better to treat the spectra simply as fingerprints (no further interpretation for the next two at least is offered here).
Pyrrolidide of 12,15-octadecadienoate (12,15-18:2) -
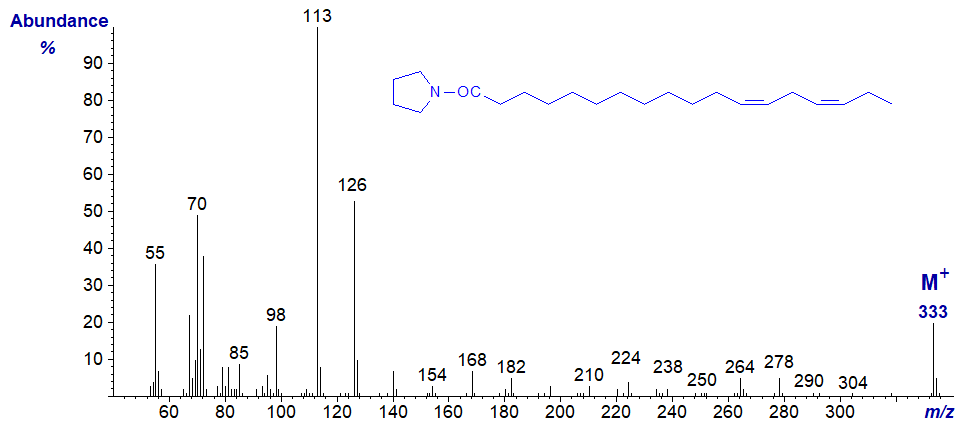
Pyrrolidide of 13,16-octadecadienoate (13,16-18:2) -
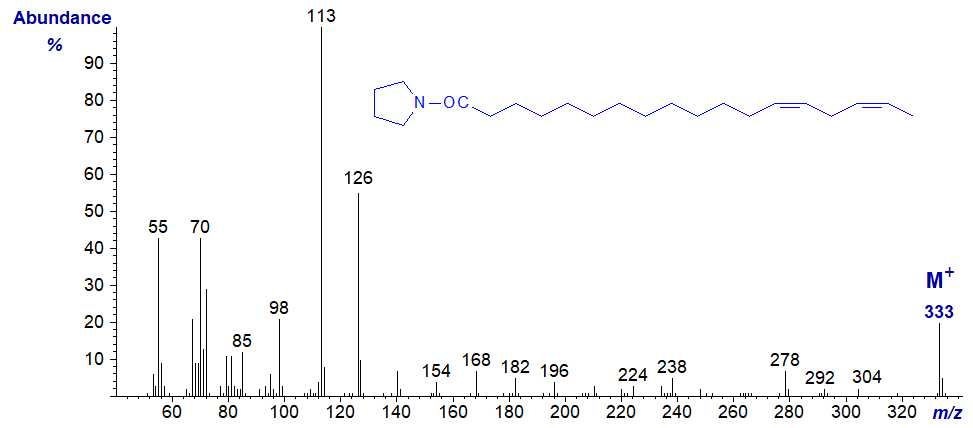
Pyrrolidide of 14,17-octadecadienoate (14,17-18:2) -
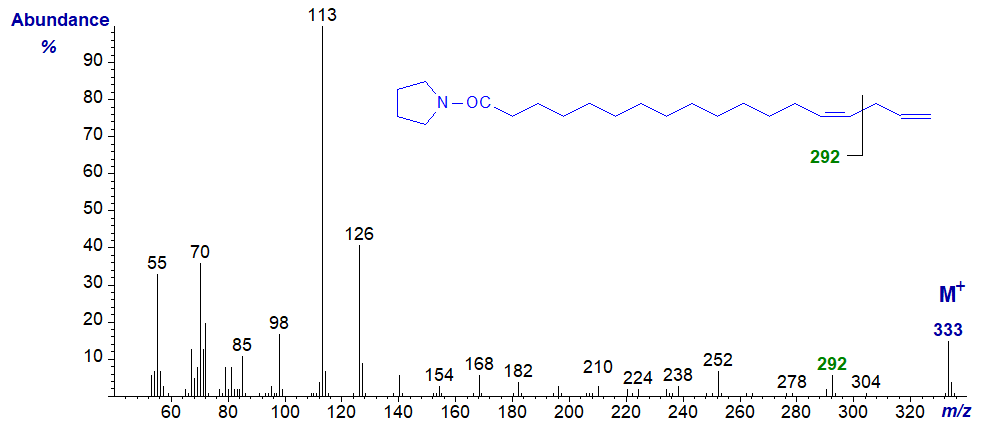
In this last spectrum, the terminal double bond is defined by the ion beta to the double bond at m/z = 292, as in the spectra of pyrrolidides of terminal monoenes.
Some Other Methylene-Interrupted Dienes
Mass spectra of pyrrolidides of methylene-interrupted dienes of different chain-lengths are of course interpreted in the same way as the next spectra show. The diagnostic ions are indicated, and it should be evident that they are the same as on the corresponding C18 analogues, as in the pyrrolidide of 9,12‑hexadecadienoate (9,12-16:2 or 16:2(n-4)) -
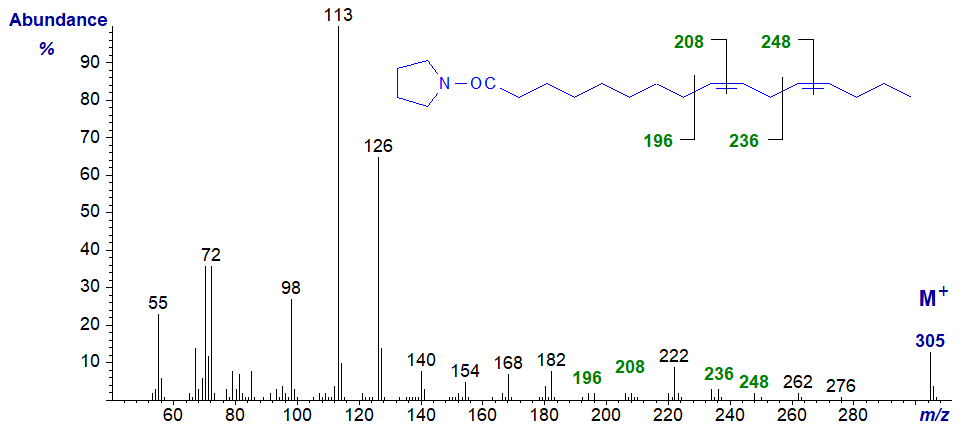
The pyrrolidide of 11,14-eicosadienoate (11,14-20:2 or 20:2(n-6)) -
Mass spectra of pyrrolidides of many more dienoic acids of various chain lengths can be found in our Archive Section, but without interpretation. Most of these have not been published elsewhere.
References
- Andersson, B.A., Christie, W.W. and Holman, R.T. Mass spectrometric determination of positions of double bonds in polyunsaturated fatty acid pyrrolidides. Lipids, 10, 215-219 (1975); DOI.
- Christie, W.W. and Holman, R.T. Synthesis and characterization of the complete series of methylene-interrupted cis,cis-octadecadienoic acids. Chem. Phys. Lipids, 1, 407-423 (1967); DOI.
I recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: November 22nd, 2023 | Contact/credits/disclaimer | |
