Mass Spectrometry of 3-Pyridylcarbinol Esters
Thia Fatty Acids
 As with
my other documents on mass spectrometry, this is a subjective account that details only
those relevant fatty acids encountered during my research activities and for which we have spectra available for illustration purposes.
The spectra described here are of synthetic fatty acids,
prepared by my friend and collaborator Professor Marcel Lie Ken Jie of Hong Kong University, and they do not occur in nature.
However, these and related hetero-atom fatty acids are being considered for therapeutic purposes and have biochemical applications.
Their mass spectra may be of interest from the standpoint of mechanistic mass spectrometry, but this is not my primary concern here.
As with
my other documents on mass spectrometry, this is a subjective account that details only
those relevant fatty acids encountered during my research activities and for which we have spectra available for illustration purposes.
The spectra described here are of synthetic fatty acids,
prepared by my friend and collaborator Professor Marcel Lie Ken Jie of Hong Kong University, and they do not occur in nature.
However, these and related hetero-atom fatty acids are being considered for therapeutic purposes and have biochemical applications.
Their mass spectra may be of interest from the standpoint of mechanistic mass spectrometry, but this is not my primary concern here.
Mass spectra of 3-pyridylcarbinol esters are described in this document, but I describe key diagnostic ions only, as general features of the spectra of these derivatives are described in the other web pages in this section. I have no spectra for DMOX or pyrrolidine derivatives, as I was not using these during my work at the time, but the spectra of methyl esters derivatives are described in a separate document. Details of the spectra were first published in -
Christie, W.W., Lie Ken Jie, M.S.F., Brechany, E.Y. and Bakare, O. Mass spectral characterization of 3-picolinyl and methyl ester derivatives of isomeric thia fatty acids. Biomed. Environm. Mass Spectrom., 20, 629-635 (1991); DOI.
In this web page, I describe spectra of mono-thia-stearates only for illustrative purposes. Information on a series of dithia-stearates is available in the published paper, but the details are only likely to be of interest to a few specialists, so they are not discussed here.
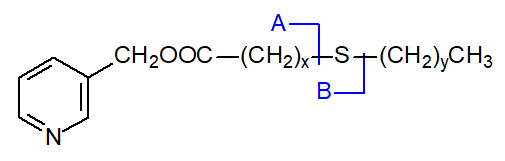 The 3-pyridylcarbinol esters
of thia fatty acids tend to give straightforward spectra with simple fragmentations that permit location of the sulfur atom.
In addition, there are the expected ions at
m/z = 92, 108, 151 and 164 for fragmentation near the carboxyl group, except in special circumstances.
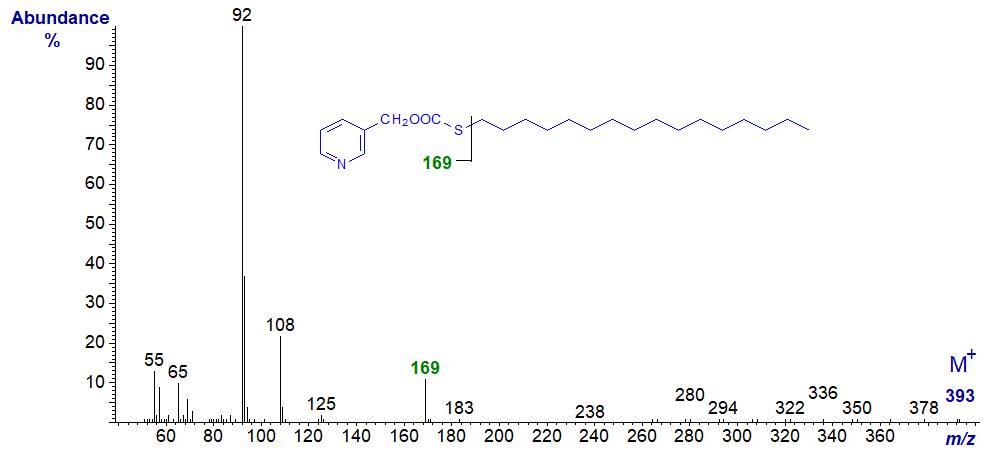
Thus, the ion for cleavage between carbons 2 and 3 in the 2-thia isomer is at m/z = 169 rather than 151;
this was once erroneously thought to be the McLafferty ion (see the web page on 3-pyridylcarbinol esters of
saturated fatty acids and Yang et al., 2006).
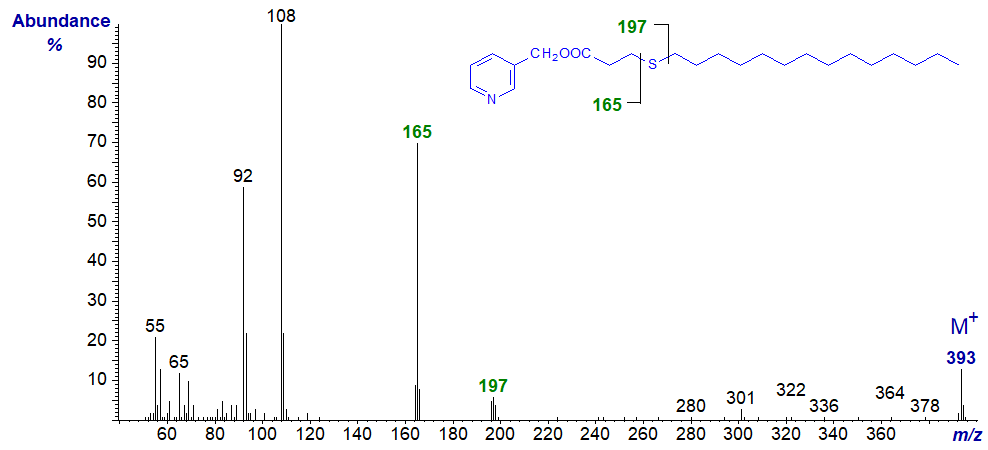
It is missing from the 4- and 6-thia-isomers, with the former because a hydrogen atom is required on carbon 4 for its formation.
I do not know why it is missing from the latter, but there is also an anomaly with the corresponding methyl ester.
The 3-pyridylcarbinol esters
of thia fatty acids tend to give straightforward spectra with simple fragmentations that permit location of the sulfur atom.
In addition, there are the expected ions at
m/z = 92, 108, 151 and 164 for fragmentation near the carboxyl group, except in special circumstances.
Thus, the ion for cleavage between carbons 2 and 3 in the 2-thia isomer is at m/z = 169 rather than 151;
this was once erroneously thought to be the McLafferty ion (see the web page on 3-pyridylcarbinol esters of
saturated fatty acids and Yang et al., 2006).
It is missing from the 4- and 6-thia-isomers, with the former because a hydrogen atom is required on carbon 4 for its formation.
I do not know why it is missing from the latter, but there is also an anomaly with the corresponding methyl ester.
In all the isomers, cleavage occurs on either side of the sulfur atom to give the two fragments containing the carboxyl end of the molecule, as shown in the figure and detailed in Table 1. The relative proportions of these ions vary from isomer to isomer, and in the early isomers, ion A tends to be more abundant, but with the 7- and later isomers, ion B is more important (often the base ion). Unusually for 3-pyridylcarbinol esters, ion A is odd-numbered until the sulfur atom is more remote from the carboxyl group. On either side of these key ions are usually ions for cleavage at successive methylene groups, i.e., 14 amu apart, although they are sometimes barely detectable. There are ions at m/z = 285 and 301 in the early spectra for which I have no explanation.
Table 1. Relative abundances (%) of ions for fragmentations adjacent to the sulfur atom in mass spectra of 3-pyridylcarbinol esters (see figure). |
|||||
| Positional isomer | Ions (%) | Positional isomer | Ions (%) | ||
|---|---|---|---|---|---|
| A | B | A | B | ||
| 2 | - | 169 (12)* | 10 | 249 (22) | 280 (100) |
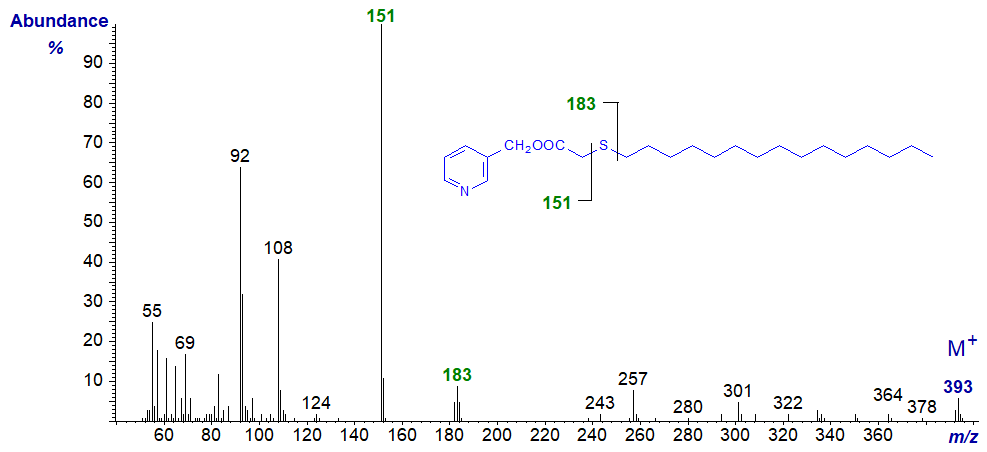
| 3 | 151 (100) | 183 (12)* | 11 | 263 (18) | 294 (100) |
| 4 | 165 (20) | 196 (5) | 12 | 277 (16) | 308 (100) |
| 5 | 179 (52) | 210 (5) | 13 | 291 (13) | 322 (100) |
| 6 | 193 (55) | 224 (18) | 14 | 305 (11) | 336 (100) |
| 7 | 207 (48) | 238 (50) | 15 | 319 (12) | 350 (100) |
| 8 | 221 (40) | 252 (77) | 16 | 333 (9) | 364 (100) |
| 9 | 235 (22) | 266 (100) | 17 | 346 (28) | 378 (100) |
| * at B1 +1 with this isomer | |||||
The mass spectra of the selected isomers that follow are offered without further comment as the main fragmentations are depicted simplistically on the figures.
3-Pyridylcarbinyl 2-thia-stearate
3-Pyridylcarbinyl 3-thia-stearate
3-Pyridylcarbinyl 4-thia-stearate
3-Pyridylcarbinyl 5-thia-stearate
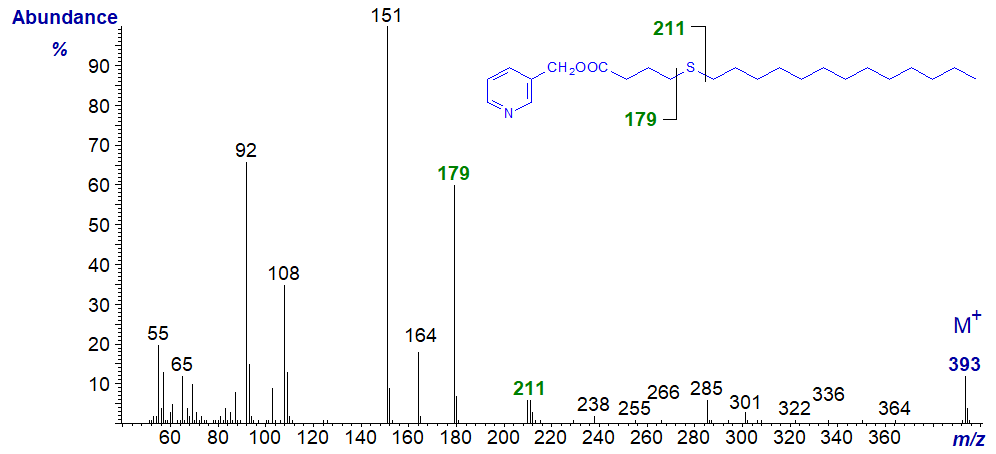
3-Pyridylcarbinyl 6-thia-stearate
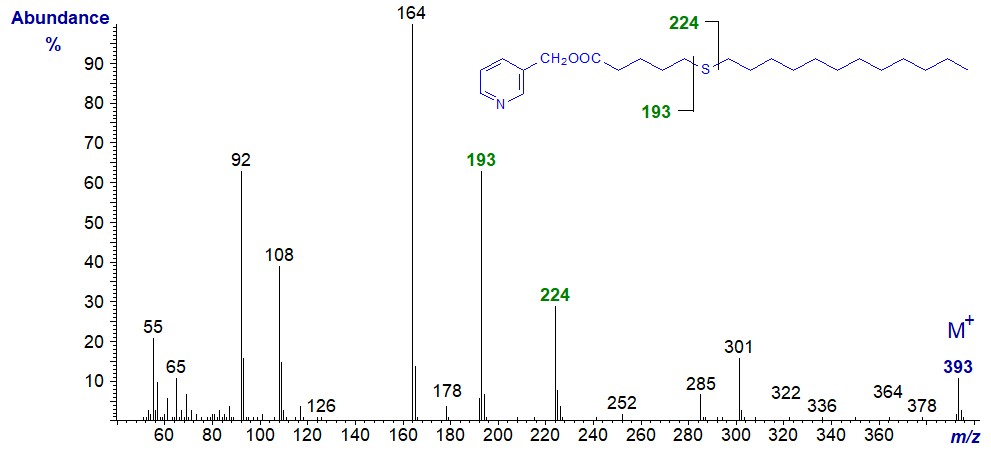
3-Pyridylcarbinyl 7-thia-stearate
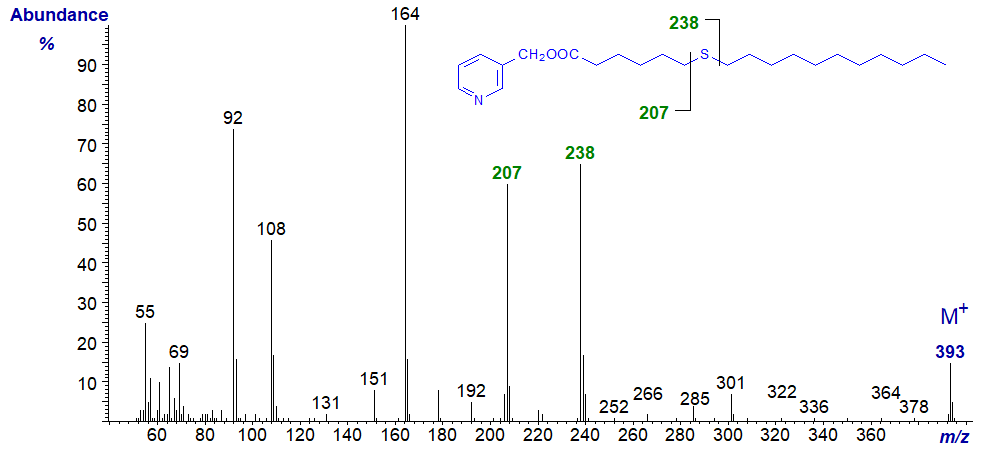
3-Pyridylcarbinyl 9-thia-stearate
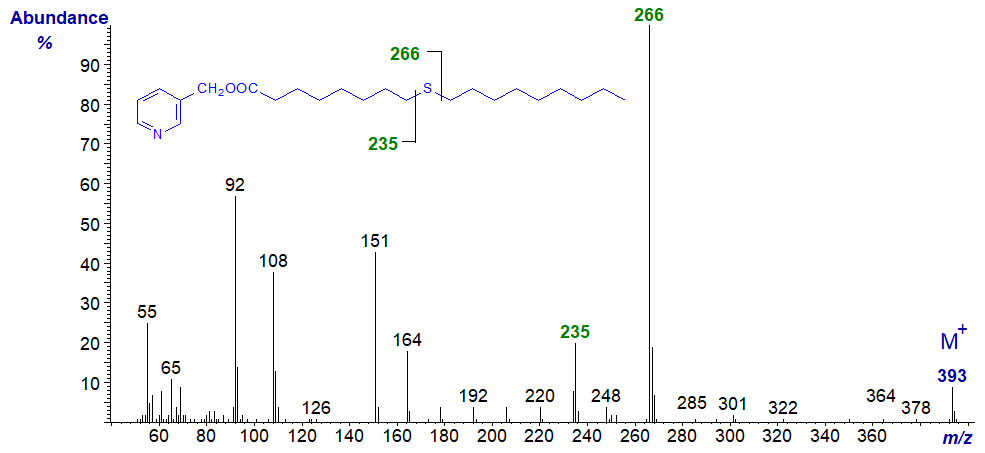
3-Pyridylcarbinyl 11-thia-stearate
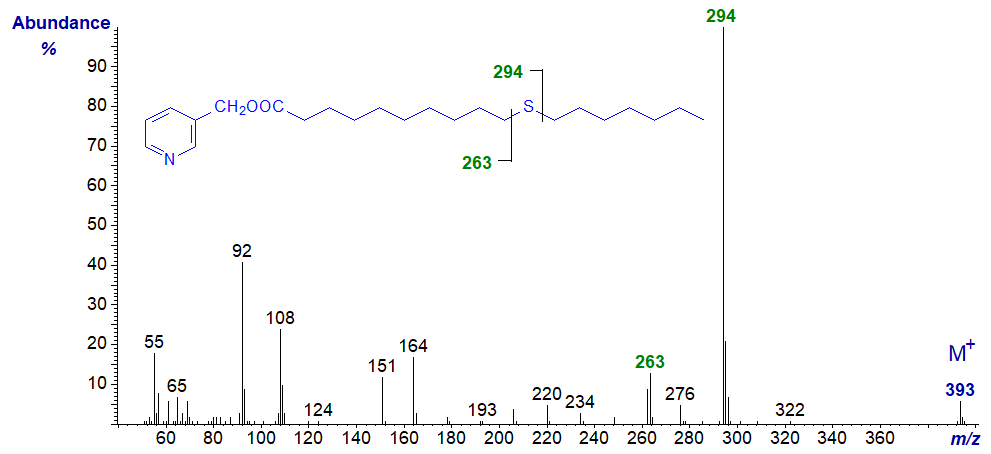
3-Pyridylcarbinyl 13-thia-stearate
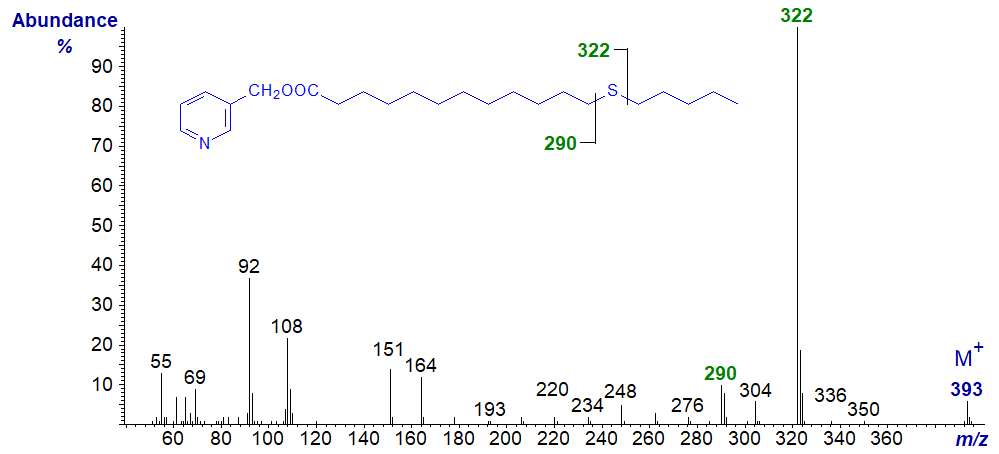
3-Pyridylcarbinyl 14-thia-stearate
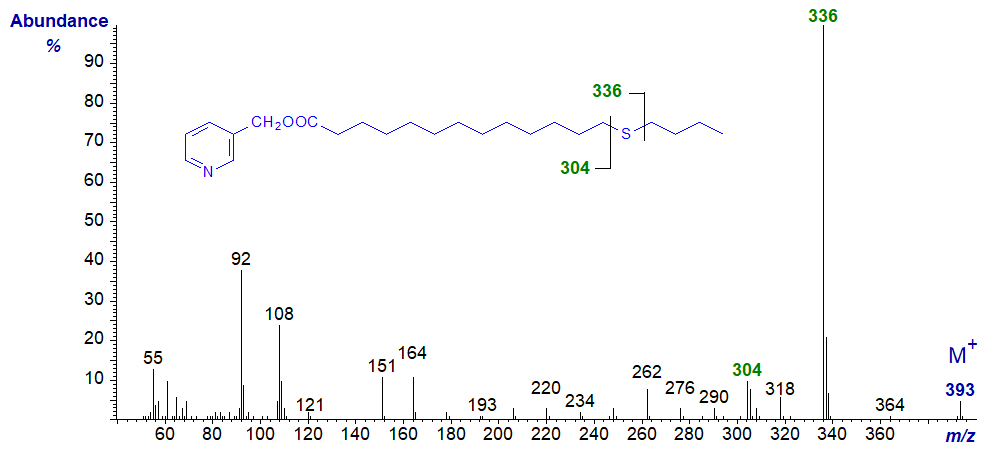
3-Pyridylcarbinyl 17-thia-stearate
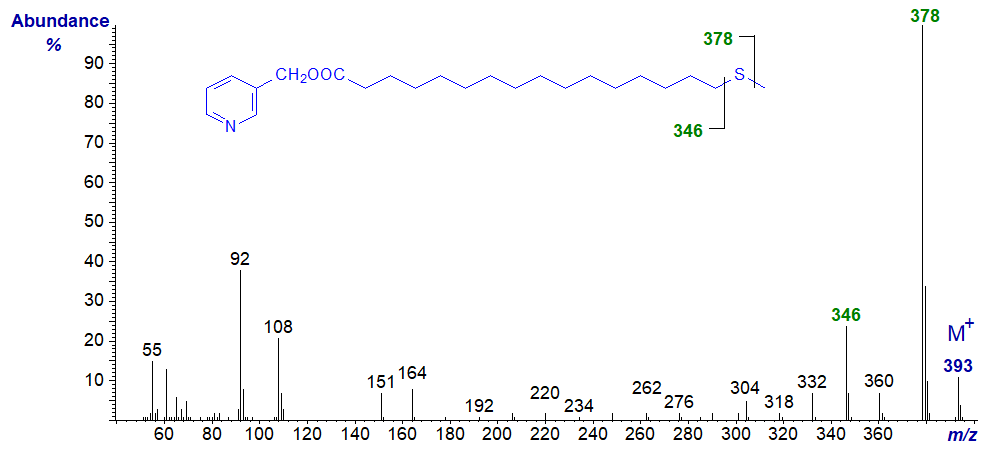
The mass spectra of 3-pyridylcarbinol ('picolinyl') esters of those thia fatty acids that are not illustrated here (including the thia-laurates (and other chain lengths) and dithia-stearates) are available in the Archive section of these web pages.
References
- Christie, W.W., Lie Ken Jie, M.S.F., Brechany, E.Y. and Bakare, O. Mass spectral characterization of 3-picolinyl and methyl ester derivatives of isomeric thia fatty acids. Biomed. Environm. Mass Spectrom., 20, 629-635 (1991); DOI.
- Yang, S., Minkler, P., Hoppel, C. and Tserng, K.-Y. Picolinyl ester fragmentation mechanism studies with application to the identification of acylcarnitine acyl groups following transesterification. J. Am. Soc. Mass Spectrom., 17, 1620-1628 (2006); DOI.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
