Mass Spectrometry of Methyl Esters
Penta- and Hexaenoic Fatty Acids
 As
cautioned in the Introduction to these documents,
the mass spectra of methyl esters obtained with electron-impact ionization afford limited
information only concerning the structures of polyunsaturated fatty acids.
Pentaenes and hexaenes as the methyl esters rarely give detectable molecular ions and even the ion
at [M−31/32]+ for loss of a methoxyl group may not be distinguishable.
Gas chromatographic retention data are then especially important as additional aids to identification, together of course
with authentic spectra for comparison.
The Introduction to our web page on tetraenoic fatty acids describes the origins of some of the
useful diagnostic ions in greater detail.
As
cautioned in the Introduction to these documents,
the mass spectra of methyl esters obtained with electron-impact ionization afford limited
information only concerning the structures of polyunsaturated fatty acids.
Pentaenes and hexaenes as the methyl esters rarely give detectable molecular ions and even the ion
at [M−31/32]+ for loss of a methoxyl group may not be distinguishable.
Gas chromatographic retention data are then especially important as additional aids to identification, together of course
with authentic spectra for comparison.
The Introduction to our web page on tetraenoic fatty acids describes the origins of some of the
useful diagnostic ions in greater detail.
While wishing to avoid duplication of my comments in my web pages for trienes and tetraenes, it is worth repeating that there are a few key ions that help to identify the common polyenoic fatty acids with methylene-interrupted unsaturation, especially those of the biologically important (n-6) and (n-3) families, though these must be interpreted with caution. Thus, an ion at m/z = 150 is characteristic for fatty acids with an n-6 terminal moiety, while one at m/z = 108 defines an n-3 terminal group (omega ion) (Holman and Rahm, 1971; Brauner et al., 1982; Fellenberg et al., 1987). Similarly, the presence of an alpha ion that contains the carboxyl group and the first two double bonds is less often recognized and tends to be small, although it often seems to occur in a quiet area of the spectrum fortunately. It is equally important for characterization, and it is discussed at greater length in the web page dealing with methyl esters of trienoic fatty acids, which contains a table of m/z values for all the common structures. A stable tropylium ion at m/z = 91 is an expected feature.
Some of the spectra depicted here will have been published somewhere in the scientific literature (and hopefully readers will point out any citations that we have missed), but others are illustrated here for the first time.
Pentaenoic Fatty Acids
The mass spectrum of methyl 5,8,11,14,17-eicosapentaenoate (20:5(n-3) or 'EPA'), the most common fatty acid in this class and arguably the most important from a biological standpoint -
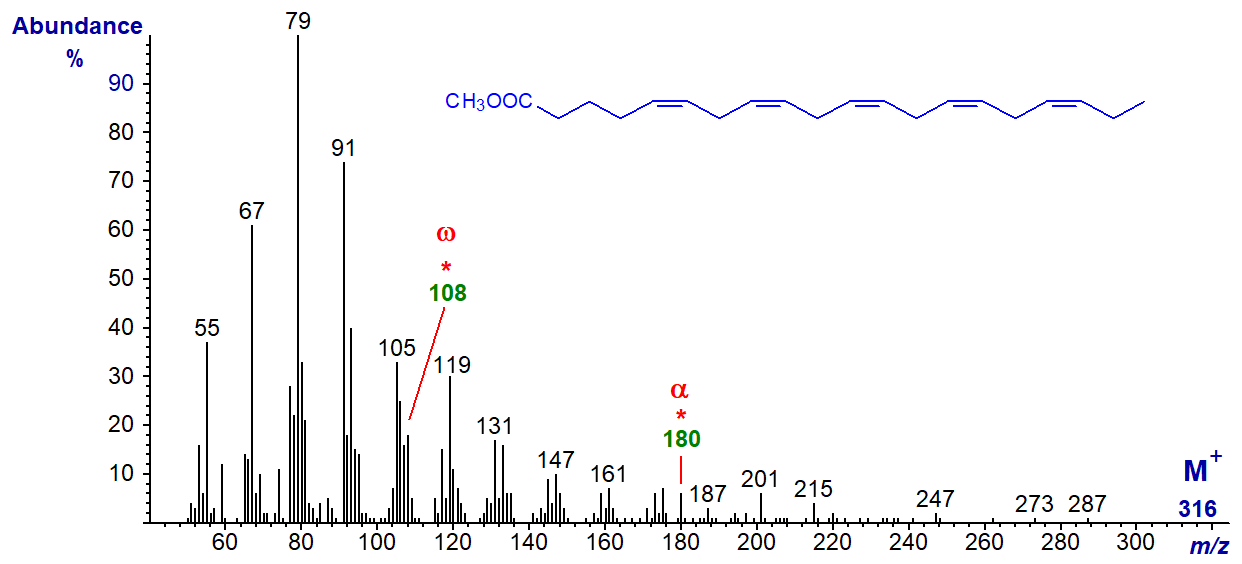
The molecular ion is just discernible with some amplification of the spectrum, but the 'diagnostic' omega ion at m/z = 108 for an n‑3 double bond is part of a cluster. The alpha ion for the Δ5,8 double bonds at m/z = 180 is small but is in a part of the spectrum that is otherwise clear. We have a spectrum for the methyl ester of 21:5(n‑3) from a fish oil on file here...
With the mass spectrum of the homologous methyl 7,10,13,16,19-docosapentaenoate (22:5(n-3)) -
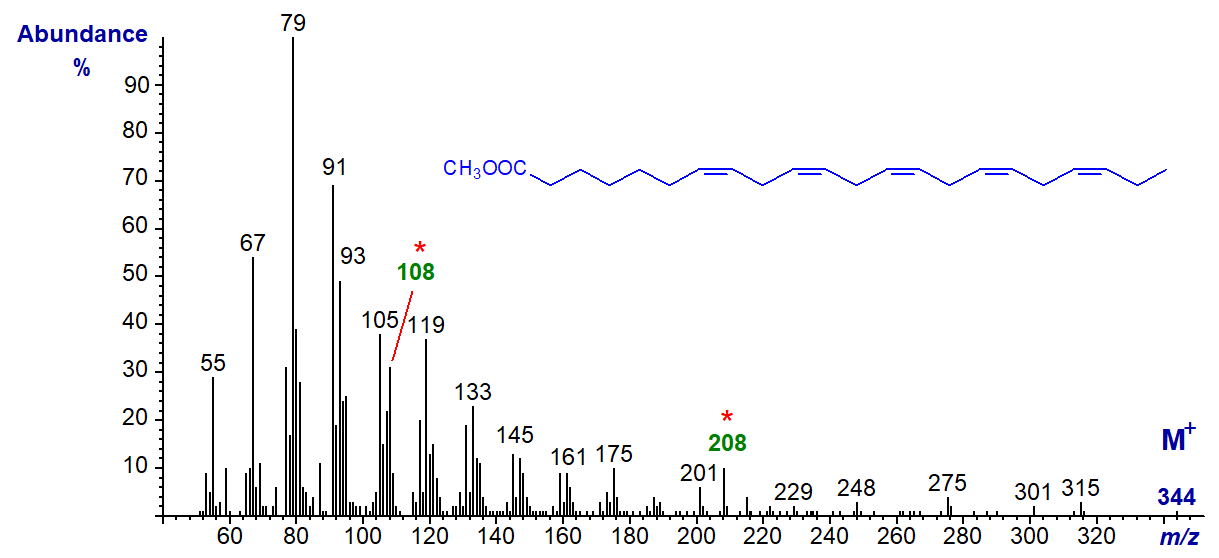
The molecular ion (m/z = 344) is very small, but the diagnostic alpha (m/z = 208) and omega (m/z = 108) ions are both evident. The spectrum of a further homologue, methyl 9,12,15,18,21-tetracosapentaenoate (24:5(n-3)), is available in our Archive page.
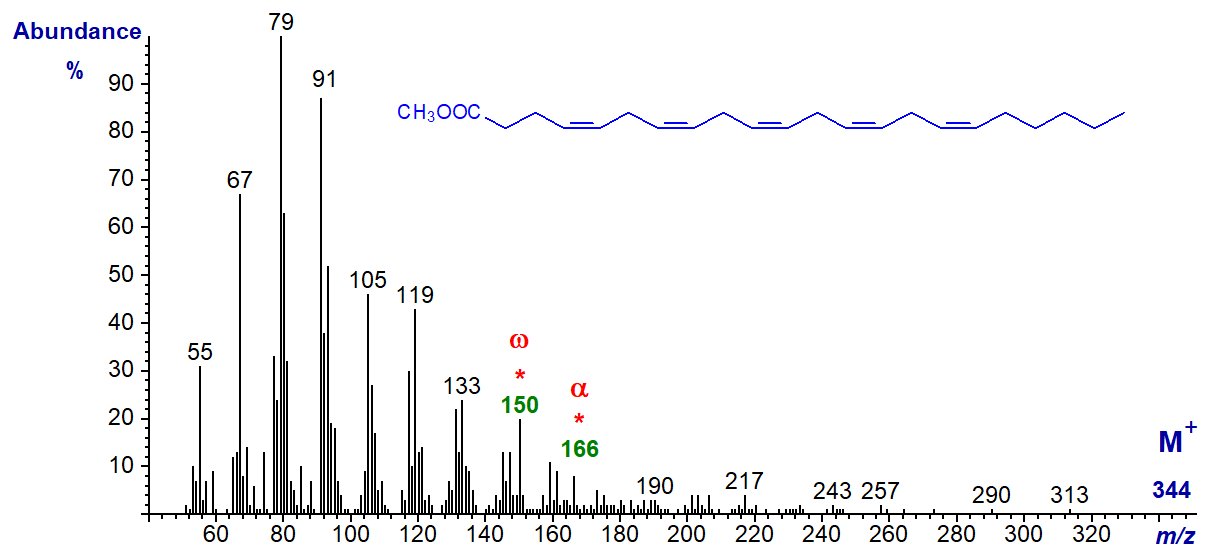
In the mass spectrum of methyl 4,7,10,13,16-docosapentaenoate or 22:5(n-6), the molecular ion is just discernible and the expected omega ion at m/z = 150 and alpha ion at m/z = 166 are seen –
3,6,9,12,15-octadecapentaenoic acid (18:5(n-3)) is occasionally found as a minor component of some marine algae and dinoflagellates (sample courtesy of Professor Otto Grahl-Nielsen), and its methyl ester has the mass spectrum –
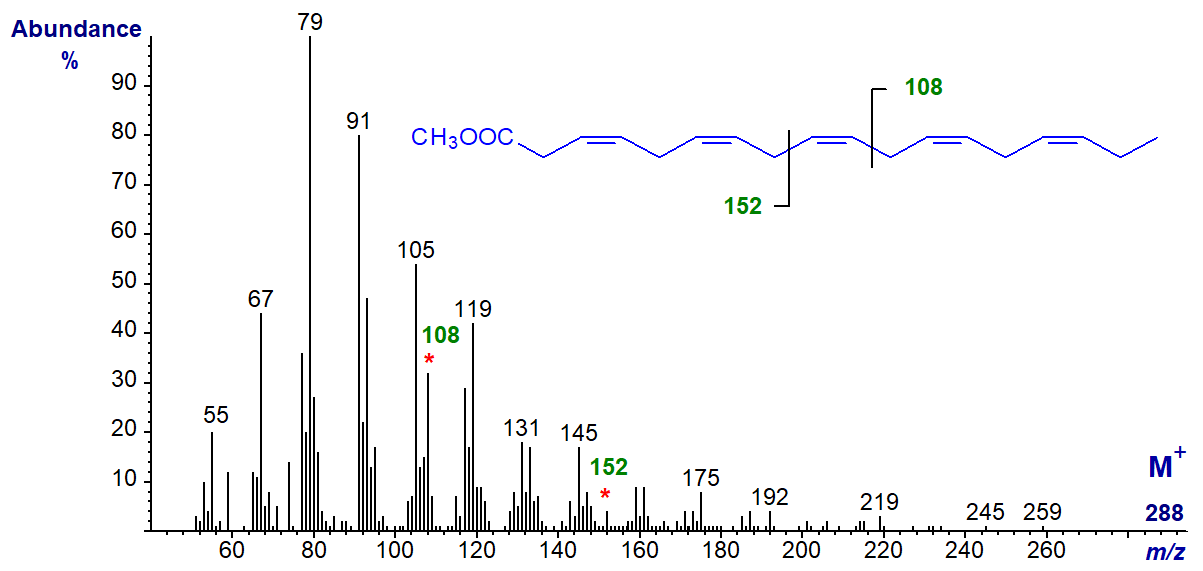
It has the expected omega ion at m/z = 108 and the alpha ion at m/z = 152, with no significant molecular ion. When preparing methyl ester derivatives in this instance, it was necessary to use the mildest conditions possible to prevent the double bond in position 3 isomerizing rapidly to position 2, with a substantial change to the spectrum. This was corroborated in a recent study, which reported that only derivatization with acidic reagents such as 5% hydrogen chloride or 1% sulfuric acid in methanol gives satisfactory results (Svetashev and Imbs, 2014).
One further C18 isomer is known, but other than an alpha ion at m/z = 180 for Δ5,8 double bonds, there is little useful structural information in the mass spectrum of the uncommon and minor fatty acid from a fish oil concentrate - methyl 5,8,11,14,17-octadecapentaenoate (18:5(n-1)) -
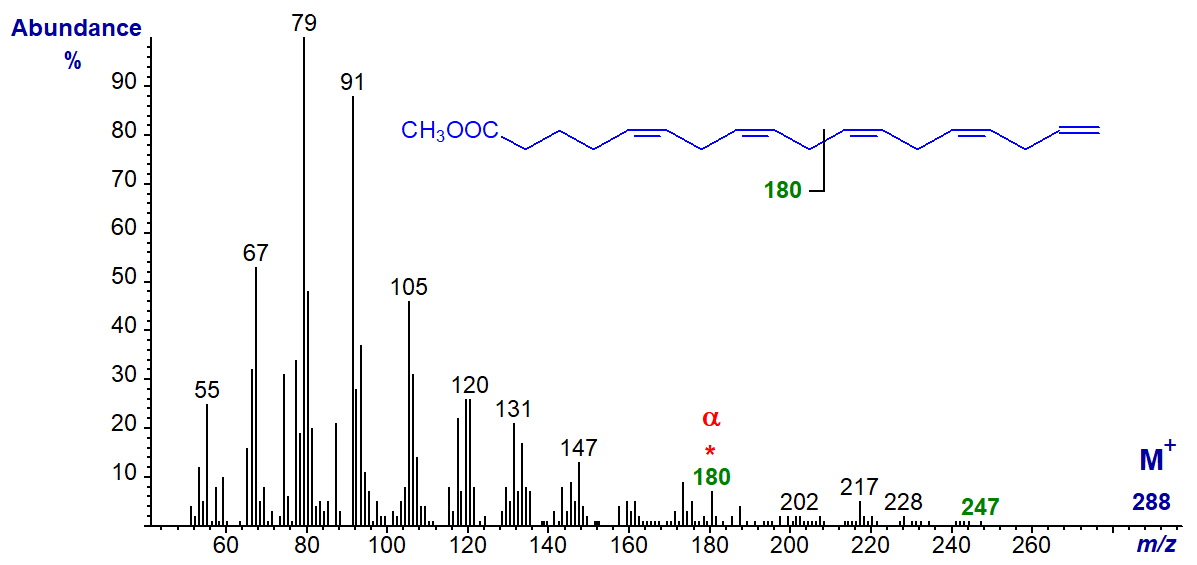
There is a small ion at m/z = 247 ([M-41]+), which may represent loss of the terminal three carbons as described for the (n-1) tetraenes.
Hexaenoic Fatty Acids
The spectrum of methyl 4,7,10,13,16,19-docosahexaenoate (22:6(n-3) or 'DHA') is undistinguished, and the molecular ion is vanishingly small, but it does have the expected omega ion at m/z = 108 and alpha ion at m/z = 166.
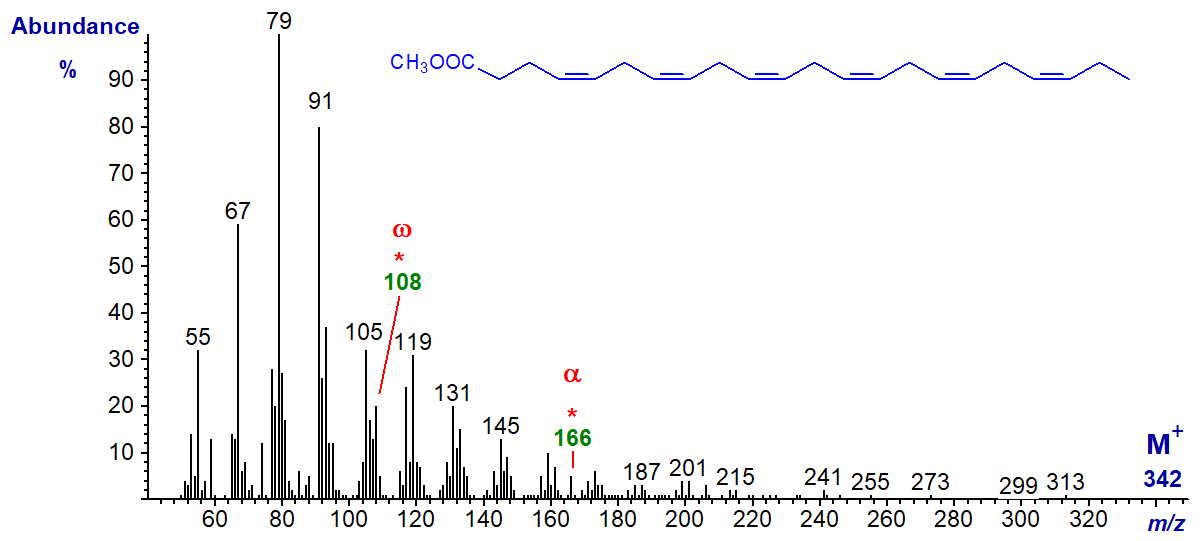
The same is true of the spectrum of methyl 6,9,12,15,18,21-tetracosahexaenoate or 24:6(n-3) (from a jelly fish, courtesy of Dr Peter Nichols), except that the alpha ion is now at m/z = 194.
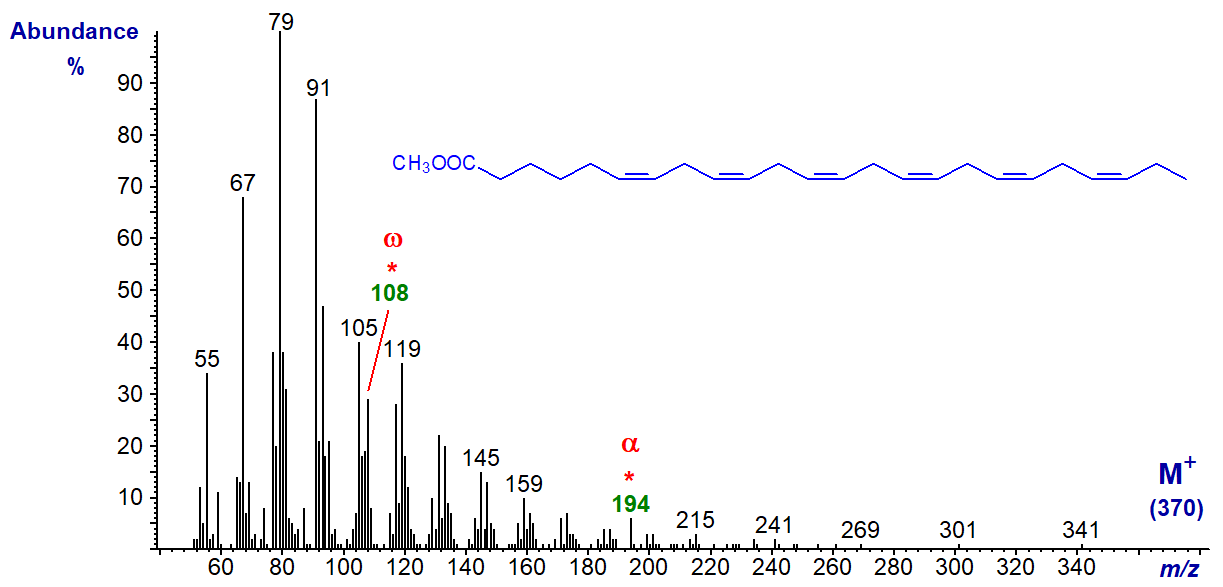
We have spectra of a few more methyl esters of polyunsaturated fatty acids on file, and they can be accessed (but without interpretation) from our Archive page. We have spectra of ethyl and other alkyl esters of such fatty acids on file, and these may be of value for comparative purposes.
References
- Brauner, A., Budzikiewicz, H. and Boland, W. Studies in chemical ionization mass spectrometry. 5. Localization of homoconjugated triene and tetraene units in aliphatic compounds. Org. Mass Spectrom., 17, 161-164 (1982); DOI.
- Fellenberg, A.J., Johnson, D.W., Poulos, A. and Sharp, P. Simple mass spectrometric differentiation of the n-3, n-6 and n-9 series of methylene interrupted polyenoic acids. Biomed. Environ. Mass Spectrom., 14, 127-130 (1987); DOI.
- Holman, R.T. and Rahm, J.J. Analysis and characterization of polyunsaturated fatty acids. Prog. Chem. Fats Other Lipids, 9, 15-90 (1971); DOI.
- Svetashev, V.I. and Imbs, A.B. Isomerization of octadecapentaenoic acid (18:5n-3) in algal lipid samples under derivatization for GC and GC-MS analysis. J. Phycol., 50, 322-327 (2014); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
