Mass Spectrometry of DMOX Derivatives
Saturated Branched-Chain Fatty Acids
DMOX derivatives have many virtues for structural characterization of fatty acids by means of electron impact mass spectrometry, and especially for locating double bonds in the aliphatic chains. They afford better resolution than the other nitrogen-containing derivatives when separated by gas chromatography. However, they are arguably not the best for locating methyl-branches in fatty acids in the most common fatty acids of this type with iso and anteiso-branches, although they have been used for the purpose by identifying ‘local intensity minima’ (Yu et al., 1988). This is one example, at least, where 3‑pyridylcarbinol esters (and pyrrolidides) are undoubtedly much better. All the spectra illustrated here were obtained incidentally from natural samples during my own research work and not as part of a systematic study, so there are some obvious gaps. Most will not have been illustrated elsewhere.
Iso- and anteiso-Methyl-Branched Fatty Acids
The reason why DMOX derivatives are less suitable for iso- and anteiso-methyl branches lies in the fact that with saturated fatty acids, a methyl group can be lost readily from the heterocyclic ring to give two series of ions, which further fragment and confound any interpretation in the higher molecular weight region of the spectrum (Hamilton and Christie, 2000). This problem is discussed further in mechanistic terms in our web page dealing with straight-chain derivatives. Indeed, difficulties can arise in the interpretation of the spectra of DMOX derivatives of all fatty acids with terminal or near-terminal functional groups. If the spectra of the two fatty acid derivatives that follow are considered simply as 'fingerprints' for comparison with authentic spectra and are taken in conjunction with GC retention data in relation to straight-chain equivalents, they will usually suffice for identification purposes.
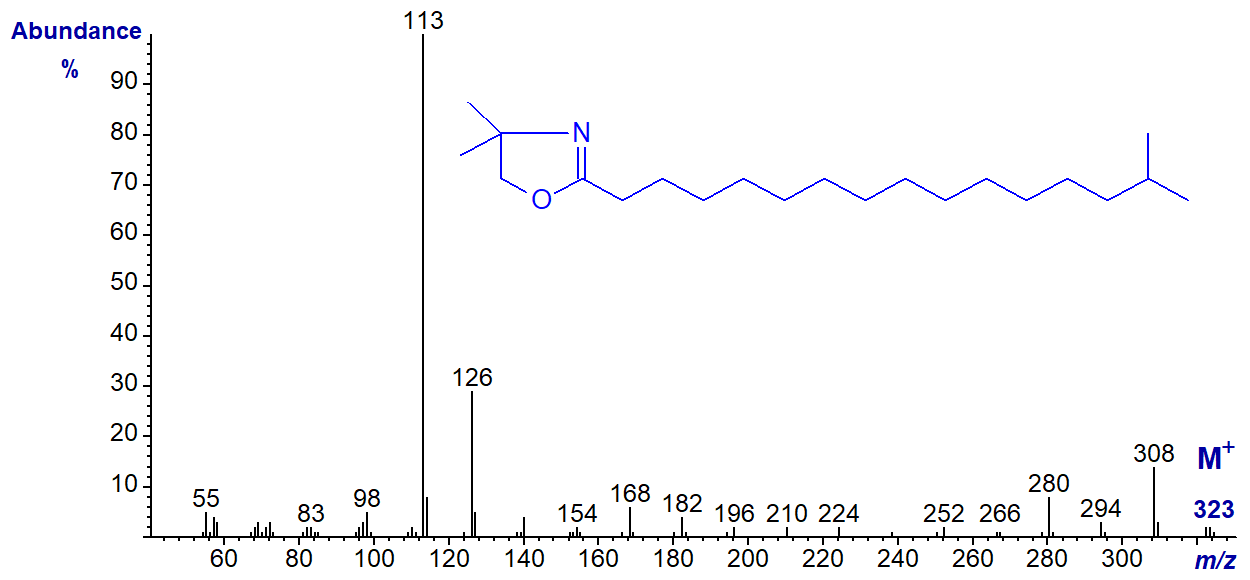
DMOX derivative of 15-methyl-hexadecanoate (iso-methyl-16:0) -
DMOX derivative of 14-methyl-hexadecanoate (anteiso-methyl-16:0) -
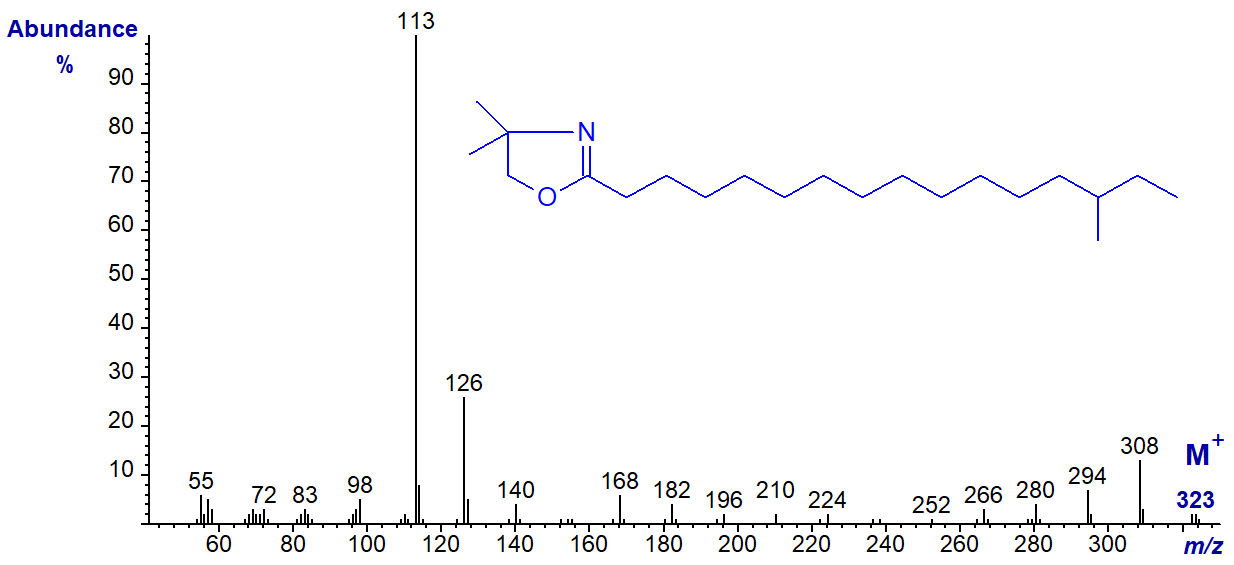
They differ in minor ways only from the spectrum of the isobaric DMOX derivative of heptadecanoate (17:0) (straight-chain and illustrated here..). Other than minor changes in ion intensity, there are no features that locate the branch point. In contrast, compare the spectra of the corresponding 3‑pyridylcarbinol esters or pyrrolidides.
With very-long-chain fatty acids with iso-methyl branches, such as 32-methyl-tritriacontanoate (iso-methyl-33:0) in our Archive pages here, the branch point can be located from a gap of 28 amu in the spectrum, but not with the comparable anteiso-isomers.
Fatty Acids with Centrally Located Methyl Branches
In the spectra of isomers where the methyl branch is more central, these can be located with relative ease by the gaps of 28 amu between key ions as for pyrrolidides and 3-pyridylcarbinol esters, although as the diagnostic ions are of relatively low abundance, interpretation can sometimes be difficult when faced with unknowns. The instrument operator can of course amplify the appropriate part of the spectrum. The spectrum of the DMOX derivative of 14-methyl-heptadecanoate is -
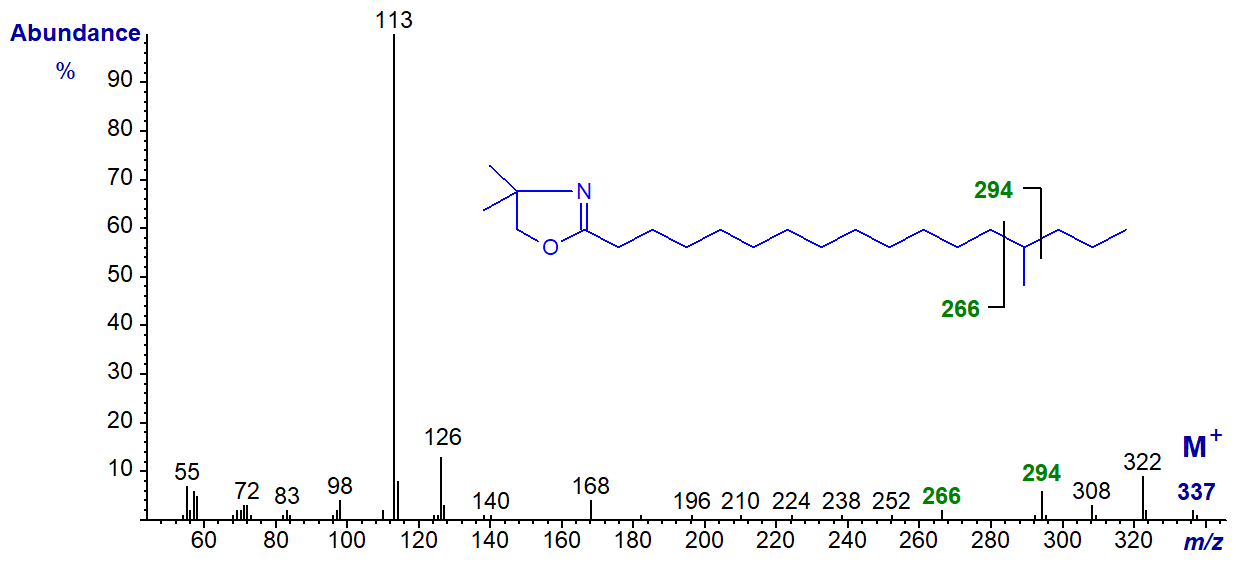
In this instance, the gap of 28 amu is between m/z = 266 and 294, for the loss of carbon 14 and the associated methyl group.
The gap of 28 amu is between m/z = 224 and 252, for the loss of carbon 11 and the associated methyl group in the spectrum of the DMOX derivative of 11-methyl-octadecanoate
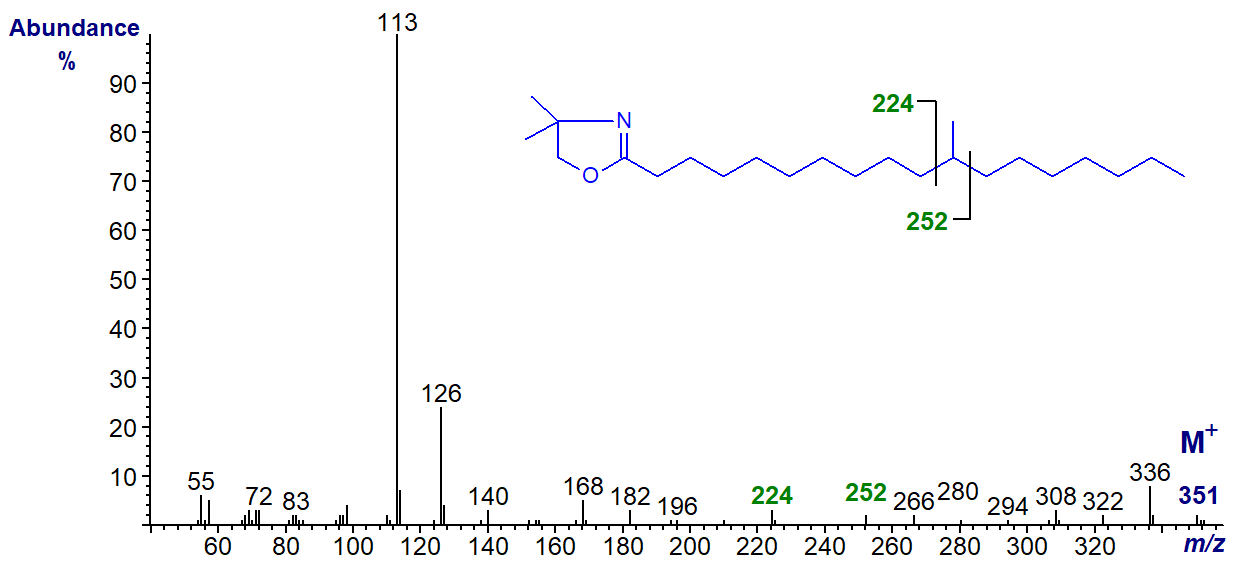
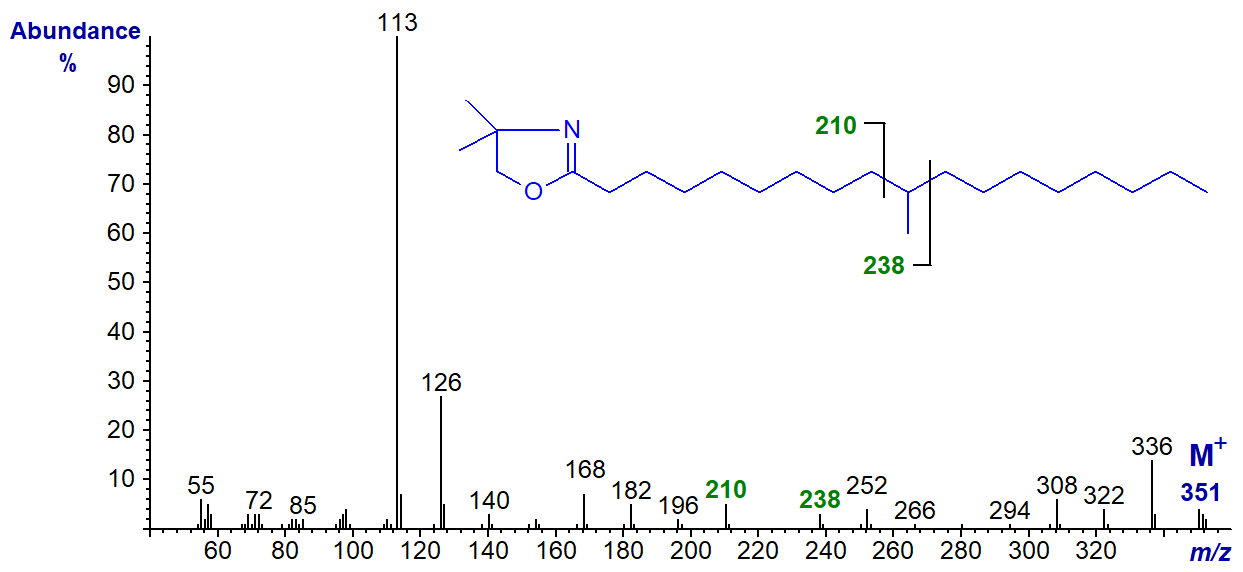
In the mass spectrum of the DMOX derivative of 10-methyl-octadecanoate (tuberculostearate), the gap of 28 amu is now between m/z = 210 and 238 -
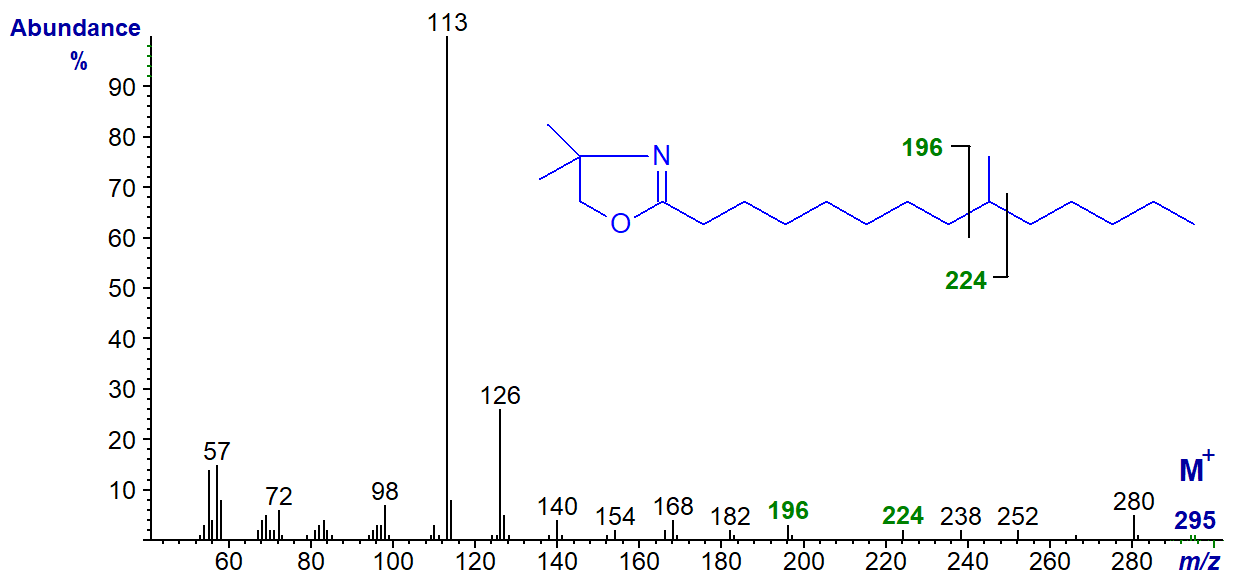
The spectrum of the DMOX derivative of 9-methyl-tetradecanoate has the gap of 28 amu between m/z = 196 and 224.
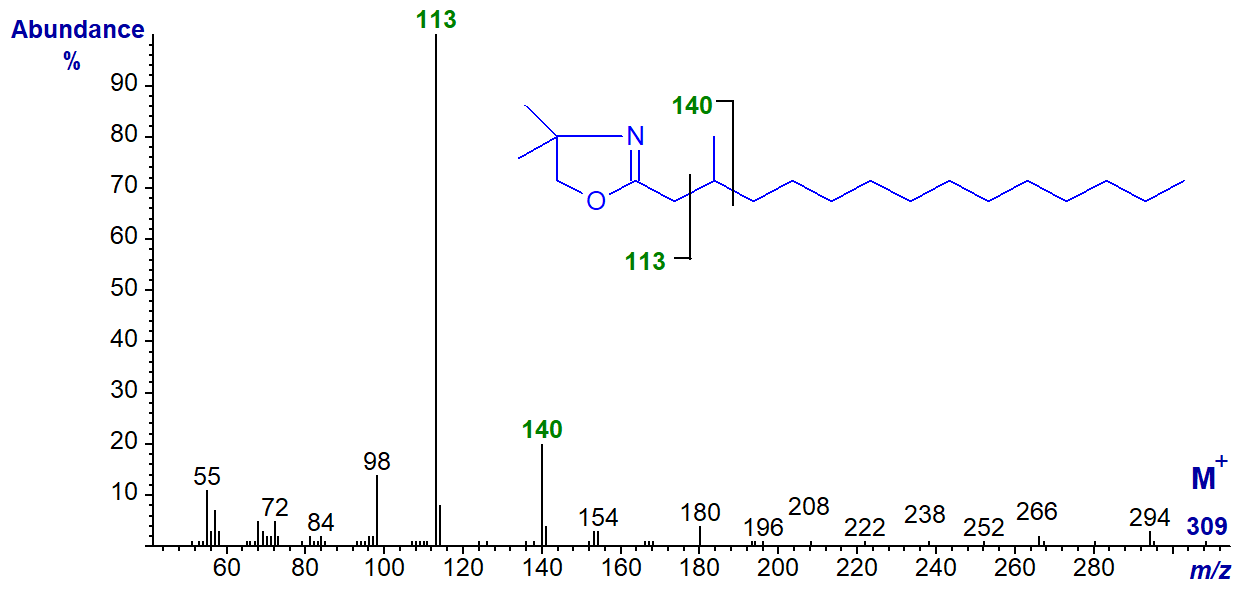
The mass spectrum of the DMOX derivative of 3-methyl-pentadecanoate is unusual if not unexpected in that the customary abundant ion at m/z = 126 is missing; it is of course shifted to m/z = 140 because of the attached methyl group and there is a gap of 27 amu between m/z = 113 to 140 to locate the branch point.
We have mass spectra on file for the DMOX derivatives of many more branched-chain fatty acids. These are illustrated without interpretation in the Archive section of these web pages.
Multi-Methyl-Branched Saturated Fatty Acids
Isoprenoid fatty acids derived from phytol are found at low levels in many animal species but especially those of marine origin. Mass spectra of the three most common of these are now illustrated. The DMOX derivative of 4,8,12-trimethyl-tridecanoate -
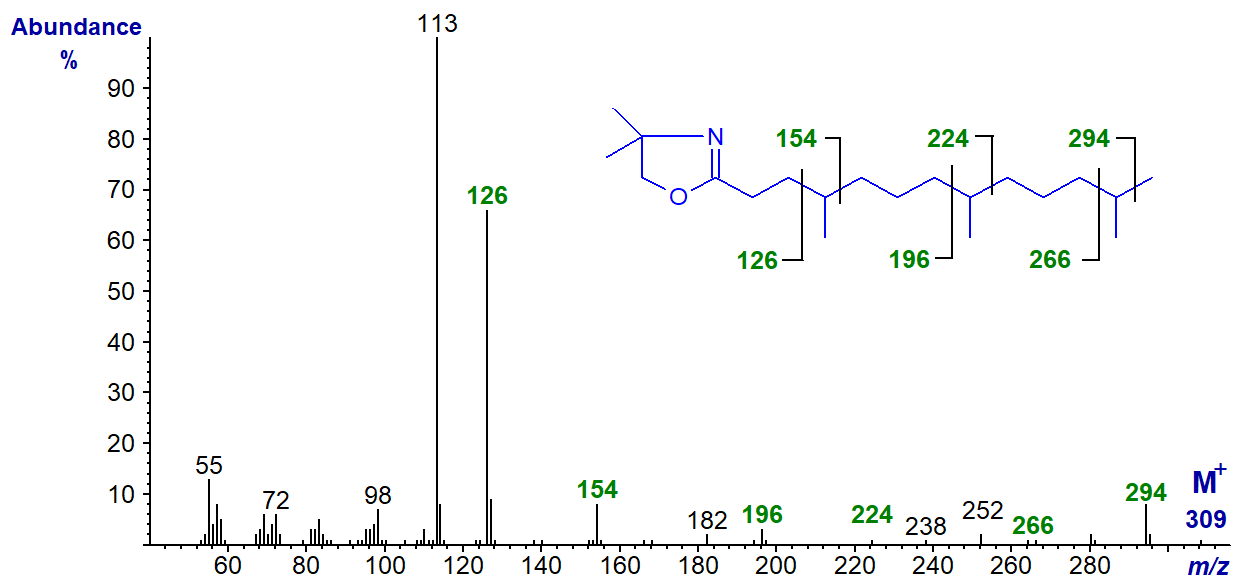
The branch points are indicated by the gaps of 28 amu between the ions marked, although that for the methyl group in position 12 is not distinct.
DMOX derivative of 2,6,10,14-tetramethyl-pentadecanoate or pristanate -
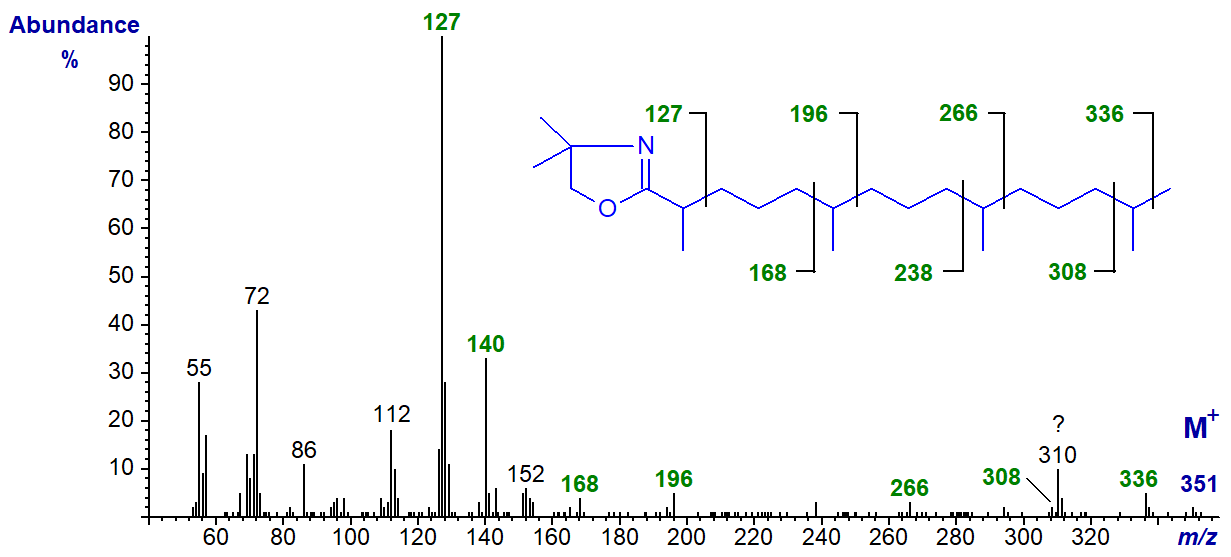
Although the diagnostic ions tend to be small, each of the branch-points can be located from the spectrum. The methyl branch in position 2 is easily seen as the expected ions at m/z = 113 and 126 are shifted upwards to m/z = 127 and 140, respectively. The remaining branches are located by the gaps between m/z = 168 and 196, 238 and 266 and 308 and 336 (the origin of the ion at m/z = 310 is not known).
DMOX derivative of 3,7,11,15-tetramethyl-hexadecanoate or phytanate -
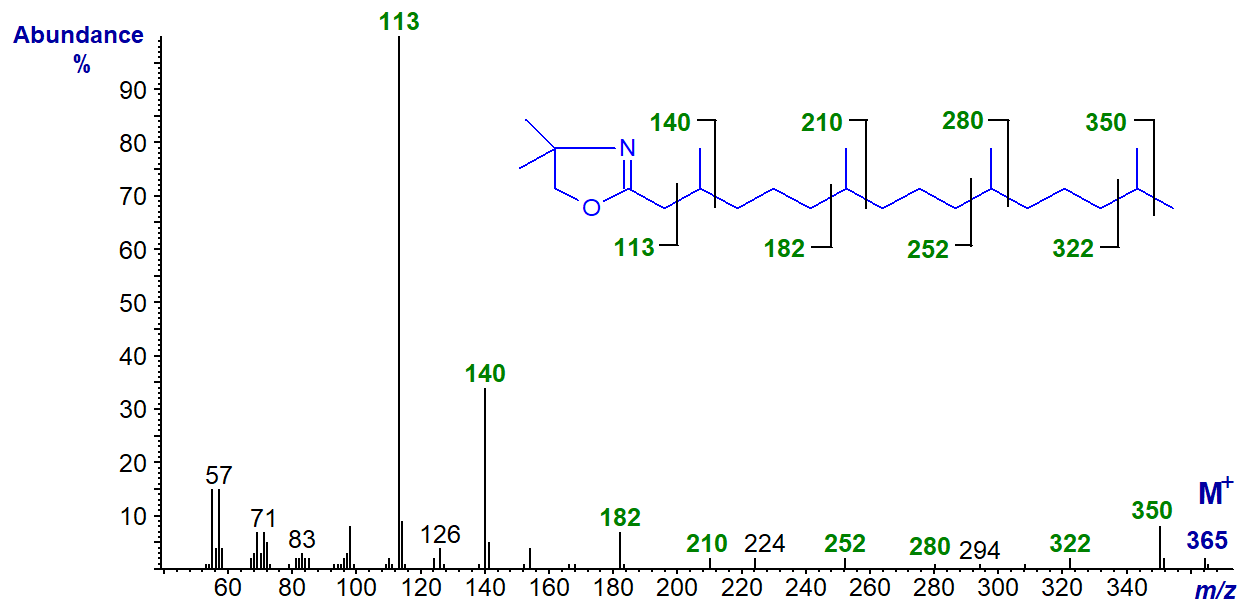
In this instance, only the ion at m/z = 126 is shifted upwards by 14 amu (cf., the spectrum for 3-methyl-15:0 above), and the gaps of 28 amu between m/z = 182 and 210, 252 and 280, and 322 and 350 serve to locate the methyl branches in positions 7, 11 and 15, respectively. It is interesting that the iso-methyl branch in these isoprenoid fatty acids can be clearly located, although this is not normally possible for mono-methyl-substituted equivalents.
We have the mass spectrum of the DMOX derivative of a non-isoprenoid dimethyl fatty acid (8,10-dimethyl-16:0) in our Archive section here... Both methyl branches are easily located.
References
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Yu, Q.T., Liu, B.N., Zhang, J.Y. and Huang, Z.H. Location of methyl branchings in fatty acids: Fatty acids in uropygial secretion of Shanghai ducks by GC-MS of 4,4‑dimethyloxazoline derivatives. Lipids, 23, 804-810 (1988); DOI.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
