Mass Spectrometry of Fatty Acid Pyrrolidides
Saturated Branched-Chain Fatty Acids
 The
mass spectra of pyrrolidide derivatives of fatty acids with anteiso- and iso-methyl branches,
i.e., those encountered most often in nature, are distinctive and can be used straightforwardly for characterization purposes,
in contrast to those of DMOX derivatives (see our web page on
'Mass spectrometry of DMOX derivatives. Saturated branched-chain fatty acids').
The first paper to be published on the topic was by Andersson and Holman (1975),
who demonstrated the ease of distinguishing iso- and anteiso-isomers in particular.
Although interpretation is usually straight forward, I much prefer to have the spectrum of an authentic sample in front of me
for comparison purposes when faced with that of an unknown fatty acid.
When methyl branches are in positions other than near the terminal end, DMOX derivatives are equally suitable for identification purposes,
while 3‑pyridylcarbinol esters are useful for characterization of all branched-chain
fatty acids.
The
mass spectra of pyrrolidide derivatives of fatty acids with anteiso- and iso-methyl branches,
i.e., those encountered most often in nature, are distinctive and can be used straightforwardly for characterization purposes,
in contrast to those of DMOX derivatives (see our web page on
'Mass spectrometry of DMOX derivatives. Saturated branched-chain fatty acids').
The first paper to be published on the topic was by Andersson and Holman (1975),
who demonstrated the ease of distinguishing iso- and anteiso-isomers in particular.
Although interpretation is usually straight forward, I much prefer to have the spectrum of an authentic sample in front of me
for comparison purposes when faced with that of an unknown fatty acid.
When methyl branches are in positions other than near the terminal end, DMOX derivatives are equally suitable for identification purposes,
while 3‑pyridylcarbinol esters are useful for characterization of all branched-chain
fatty acids.
Our web page on mass spectrometry pyrrolidides of saturated fatty acids contains more introductory and mechanistic information, together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.). To illustrate the effect of the position of the branch points on fragmentation here, it has been necessary to choose examples of fatty acids of differing chain-lengths because of the limited range of material available to us from natural sources. Few if any of the following spectra have been published formally elsewhere.
Iso- and anteiso-Methyl-Branched Fatty Acids
Those branched-chain fatty acids most often encountered in nature have iso- or anteiso-methyl branches. The mass spectrum of the pyrrolidide of 13‑methyl-tetradecanoate (iso isomer) -
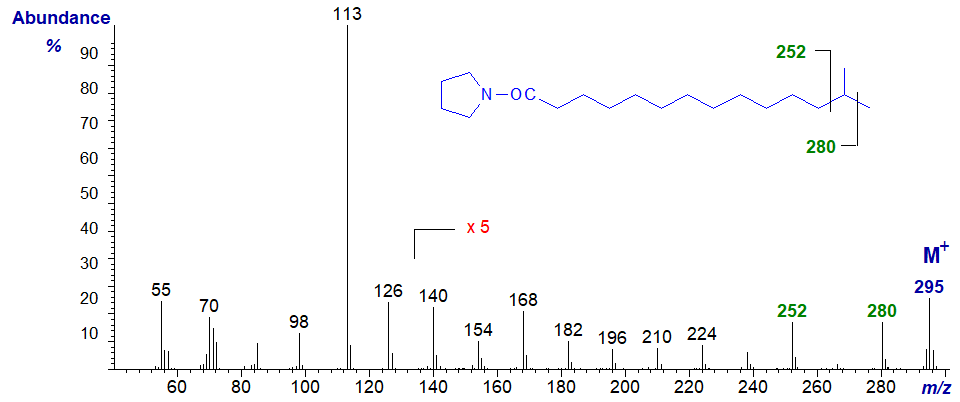
The methyl branch is easily located by the gap of 28 amu between m/z = 252 and 280 for loss of carbon 13 in the chain with its methyl group. In this and most of the following spectra, all the ions in the higher mass range have been magnified five-fold relative to the base ion for ease of viewing and to aid the interpretation.
Similarly, in the mass spectrum of the pyrrolidide of 12-methyl-tetradecanoate (anteiso isomer) -
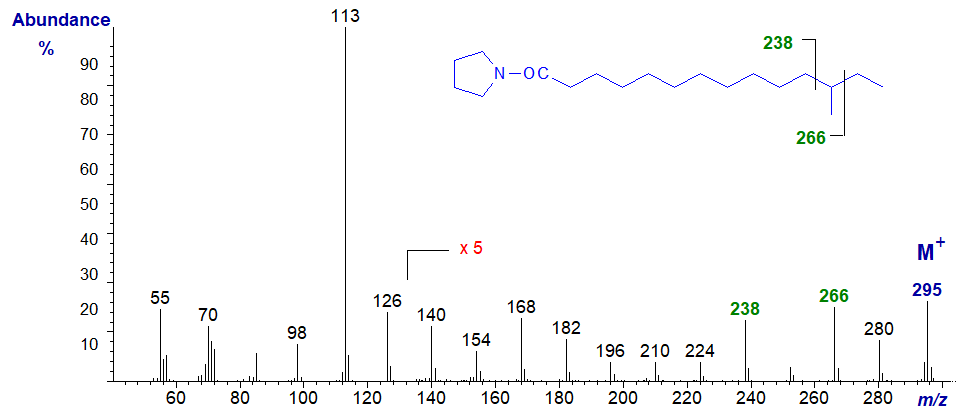
- the gap of 28 amu is now shifted downwards as expected to between m/z = 238 and 266 for loss of carbon 12 and its methyl group.
Fatty Acids with Centrally Located Methyl Branches
The mass spectrum of the pyrrolidide of 17-methyl-tetracosanoate -
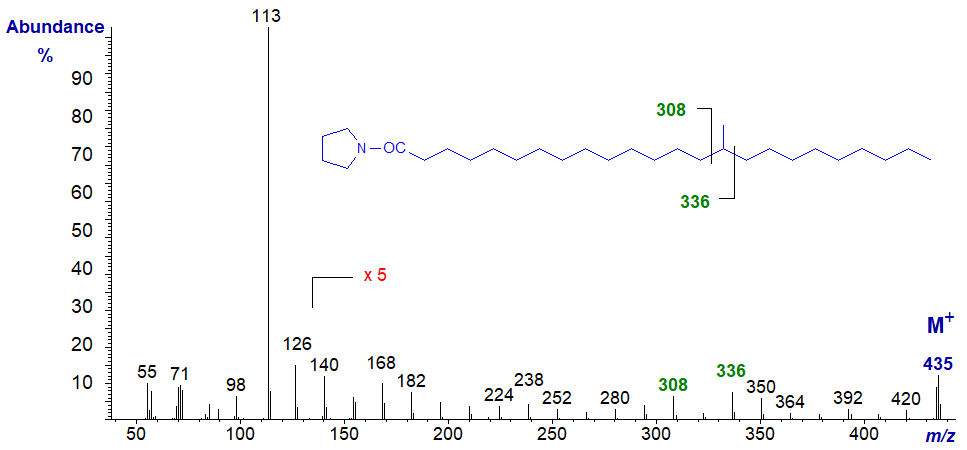
- the diagnostic gap of 28 amu is between m/z = 308 and 336 for loss of carbon 17 and its methyl group.
The mass spectrum of the pyrrolidide of 14-methyl-heptadecanoate -
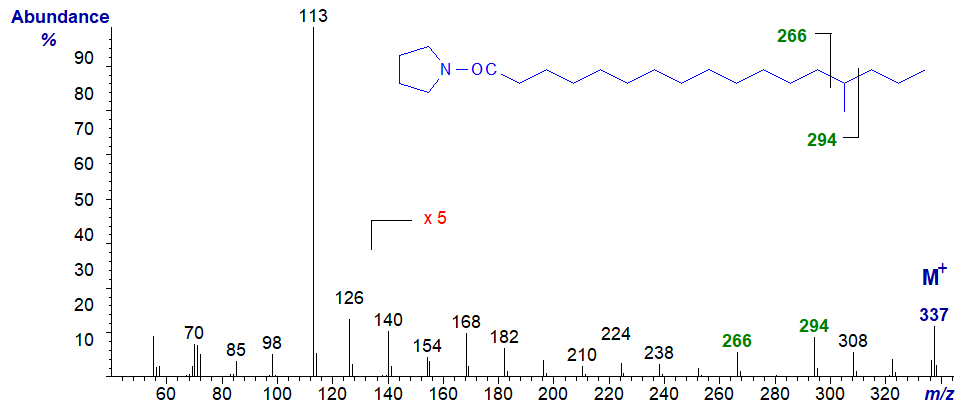
- now the diagnostic gap of 28 amu is shifted to between the ions at m/z = 266 and 294.
The mass spectrum of the pyrrolidide of 11-methyl-octadecanoate -
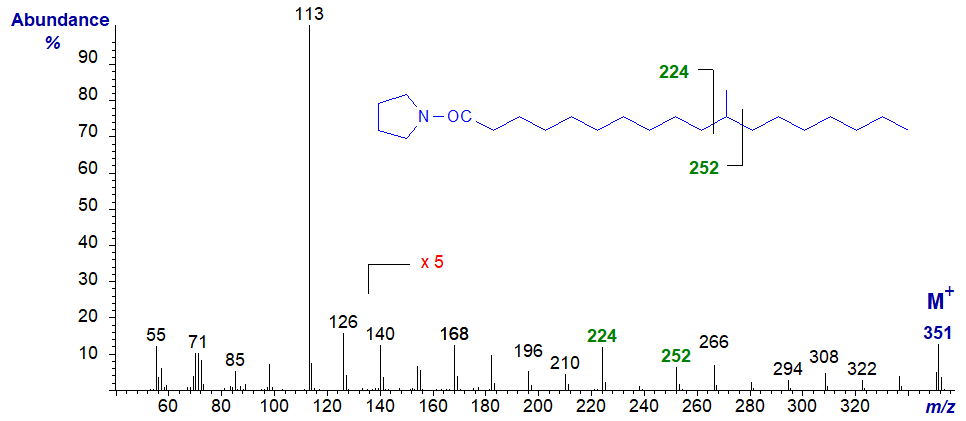
- the diagnostic gap of 28 amu is shifted to between the ions at m/z = 224 and 252.
The mass spectrum of the pyrrolidide of 9-methyl-tetradecanoate -
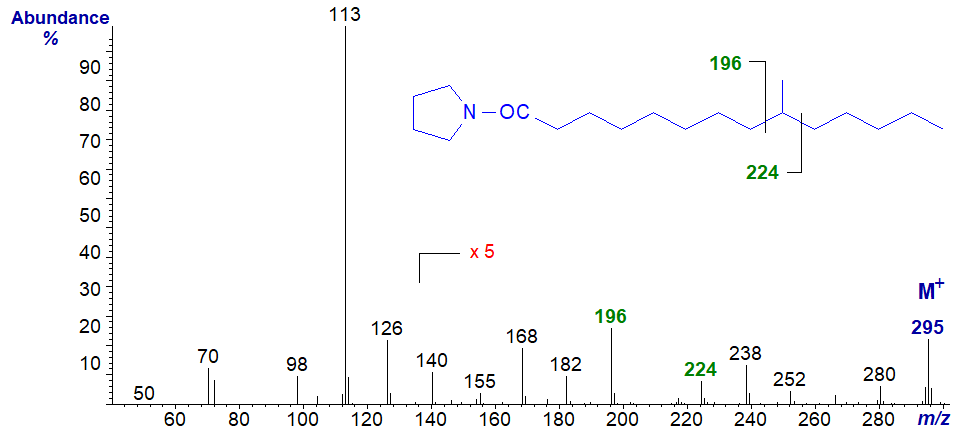
- as expected, the diagnostic gap of 28 amu is between the ions at m/z = 196 and 224.
The mass spectrum of the pyrrolidide of 3-methyl-tetradecanoate -
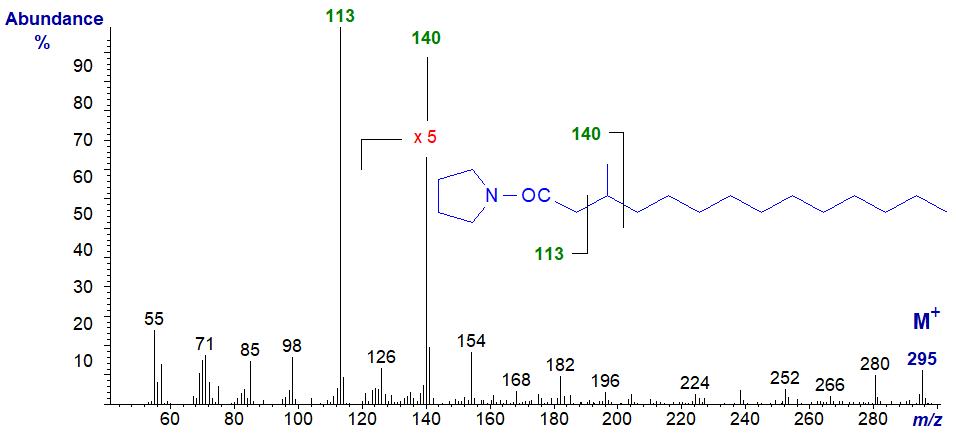
The gap of 27/28 amu for carbon-3 and its associated methyl group is between m/z = 113 and 140, and the usual ion at m/z = 126 can only be seen because of the considerable magnification.
We have mass spectra on file for pyrrolidides of many more branched-chain fatty acids, and these can be found (but without interpretation) in our Archive web page.
Multi-Methyl-Branched Fatty Acids
An unusual dimethyl-branched fatty acid, 8,10-dimethyl-hexadecanoate, has been found in a sponge and the mass spectrum of its pyrrolidine derivative is -
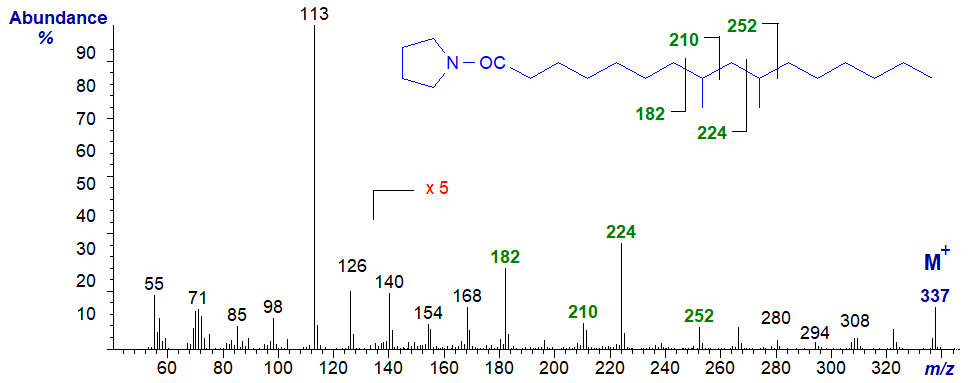
The methyl groups are located by the two gaps of 28 amu as indicated on the spectrum.
The most common multi-methyl-branched fatty acids encountered in nature are the isoprenoid compounds derived from phytol, such as 4,8,12‑trimethyltridecanoic acid, which is often encountered as a minor component of marine oils, and the mass spectrum of its pyrrolidide is illustrated next. Again, each of the methyl groups is located by the gaps of 28 amu as indicated. The last one is not clear in this instance, so it is again useful to have an authentic spectrum for comparison.
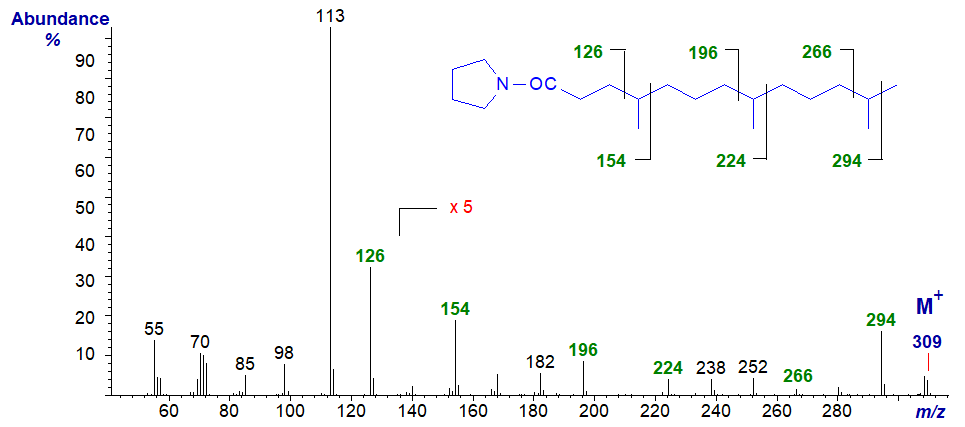
Finally, the best known of such acids is probably phytanic (3,7,11,15-tetramethyl-hexadecanoic) acid, and the mass spectrum of its pyrrolidide is -
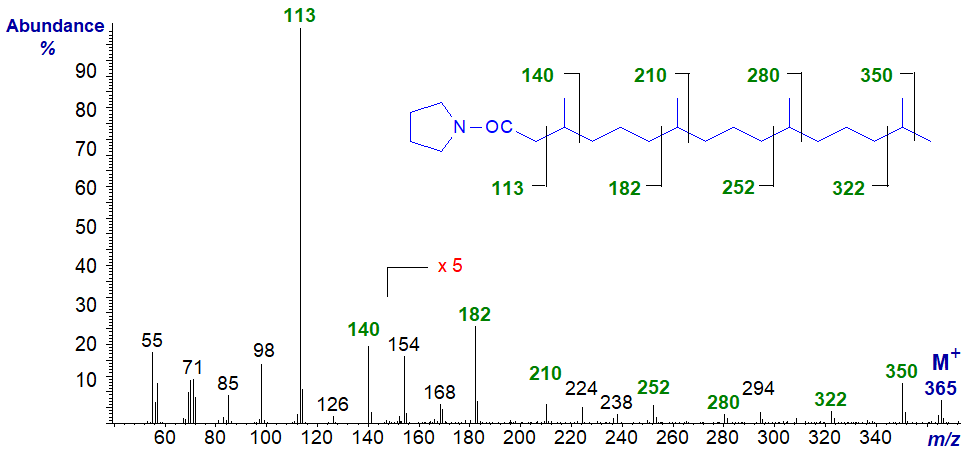
The gaps of 27/28 amu between m/z = 113 and 140, 182 and 210, 252 and 280, and 322 and 350 locate the methyl branches in positions 3, 7, 11 and 15, respectively. In this and the previous spectrum, each ion group is separated from the next by 70 amu.
References
- Andersson, B.A. and Holman, R.T. Mass spectrometric localization of methyl branching in fatty acids using acylpyrrolidines. Lipids, 10, 716-718 (1975); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
