A Beginner's Guide to Mass Spectrometry of Fatty Acids
Part 2. 3-Pyridylcarbinol, 4,4-Dimethyloxazoline and Pyrrolidine Derivatives
In Part 1 of this topic, I used an analogy in which mass spectrometry was compared to demolishing and re-assembling a brick wall. If we take it apart a few bricks at a time, it is possible to reassemble it easily; we will know the correct dimensions and where any door or window should be placed. With a fatty acid derivative, in comparison, we need to use the fragment ions to confirm that it is indeed a fatty acid, determine the molecular weight and then locate any double bonds or other functional groups. Methyl esters give satisfactory identifications in a limited range of circumstances only, and most often in simple samples where no unusual fatty acids are expected. However, there are invaluable alternative derivatives, all of which contain nitrogen atoms close to the carboxyl group. With such compounds the nitrogen atom carries the charge when the molecule is ionized in the mass spectrometer, rather than the aliphatic chain (as with methyl esters). Rather uniform fragmentation then occurs along the aliphatic chain, and with a little experience, it is easy to pick out functional groups such as double bonds, methyl branch points or cyclic structures. Many spectra are available for comparison purposes in this website in the various tutorials and in the Archive sections, and most of these spectra are not available in commercial computerized data bases.
Three types of fatty acid derivative have been used most often for structure determination: in order of the original publications, acyl pyrrolidides, 3‑pyridylcarbinol (incorrectly termed ‘picolinyl’ in most publications) esters and 4,4-dimethyloxazoline (DMOX) derivatives.
Pyrrolidides were the first derivative of this type to be described in 1971, but they have been overtaken by alternatives, probably because their chromatographic properties are less than ideal. 3-Pyridylcarbinol esters have better mass spectrometric properties than pyrrolidides, and DMOX derivatives are better for analysis by gas chromatography (GC), so these two are now favoured in most laboratories, but pyrrolidides derivatives should not be forgotten. Alternative derivatives have occasionally been proposed, but they are unlikely to be accepted unless a substantial body of sample spectra is made available for comparison or reference purposes.
In addition to the review articles cited in Part 1 of this topic, definitive reviews on the use of pyrrolidides [1], 3-pyridylcarbinol esters [2] and DMOX derivatives [3] have been published. Detailed protocols for the preparation of these derivatives are available here..
Please note that I have not considered the use of mass spectrometry for quantitative analysis of fatty acids here. Then, quite different problems arise, and methyl ester derivatives may be as good as any other for the purpose. I will leave that topic to someone else to discuss, but readers should be aware that GC with flame-ionization detection is by far the simplest and most robust approach to quantitative analysis [4].
3-Pyridylcarbinol (‘picolinyl’) Esters
3-Pyridylcarbinol esters are not as popular now as they once were, in part because their preparation can be tedious, but they remain a good choice for structural analysis of fatty acids. To illustrate this, the mass spectrum of the 3-pyridylcarbinol ester of γ-linolenic acid (6,9,12‑18:3 or 18:3(n-6)) is illustrated -
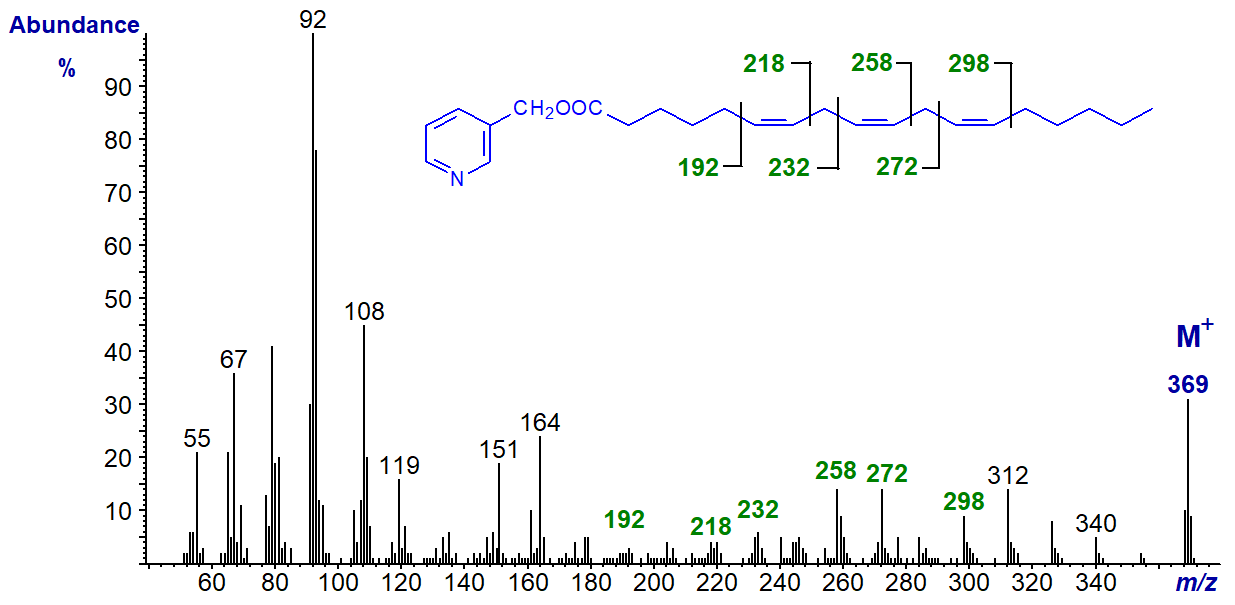
There are large ions at m/z = 92, 108, 151 and 164, which contain the pyridine ring and various elements of the carboxyl group and serve mainly to indicate that the compound is indeed a 3‑pyridylcarbinol ester. To locate functional groups such as the double bonds here, it is necessary to consider the higher molecular weight region of the spectrum. There is an abundant molecular ion (m/z = 369), which is always odd-numbered and is useful confirmation that we have a C18 fatty acid with three double bonds. Then there is a uniform series of ions 14 atomic mass units (amu) apart, representing loss of each successive methyl and methylene group from the terminal end of the molecule, until we reach the ion at m/z = 298. There is a gap of 26 amu for the carbons constituting the terminal double bond to m/z = 272, a further gap of 14 amu for the methylene group at carbon-11, then another gap of 26 amu between m/z = 234 and 258, a gap of 14 amu for the methylene group at carbon-8, and so forth.
The double bond nearest to the carboxyl group is not always easily spotted from first principles, but with a little experience it is not too difficult to define. Of course, it always helps to have access to spectra of standards for comparison purposes as in the Archive pages of this website.
After checking the ions for the pyridine ring, spectra of all other 3-pyridylcarbinol esters are thereafter interpreted in the same way, by starting with the molecular ion and working backwards one methylene group at a time until a functional group is reached. Thus, if there is a methyl branch, for example, there will be a gap of 28 amu for loss of -CH- with its attached methyl group.
3-Pyridylcarbinol esters have most often been prepared from unesterified fatty acids, and if such methods are to be used, it is necessary to first hydrolyse an intact lipid sample or methyl ester. The original method for preparation of the derivative involved dissolving the free fatty acid in an excess of thionyl chloride to form the acid chloride, which was then reacted with a 1% solution of 3-hydroxymethylpyridine in acetonitrile to form the 3‑pyridylcarbinol ester for direct analysis by GC-MS [5]. Alternatively, a mild quantitative method may be preferred that involves formation of an imidazolide by reacting the fatty acid with 1,1'-carbonyldiimidazole in dichloromethane prior to reaction with the 3-pyridylcarbinol reagent in triethylamine in the presence of 4‑pyrrolidinopyridine as a catalyst [6]. A newer method now permits direct preparation from methyl esters or intact lipids with 3-pyridylcarbinol in tetrahydrofuran and potassium tert-butoxide as catalyst [7], but again see practical details here...
4,4-Dimethyloxazoline (DMOX) Derivatives
DMOX derivatives have good chromatographic and mass spectrometric properties, and they can be prepared in a relatively simple one pot reaction, both from unesterified fatty acids or those in ester form, so they are now the most widely used derivative for structural analysis of fatty acids. The mass spectrum of the DMOX derivative of oleic acid is illustrated next -
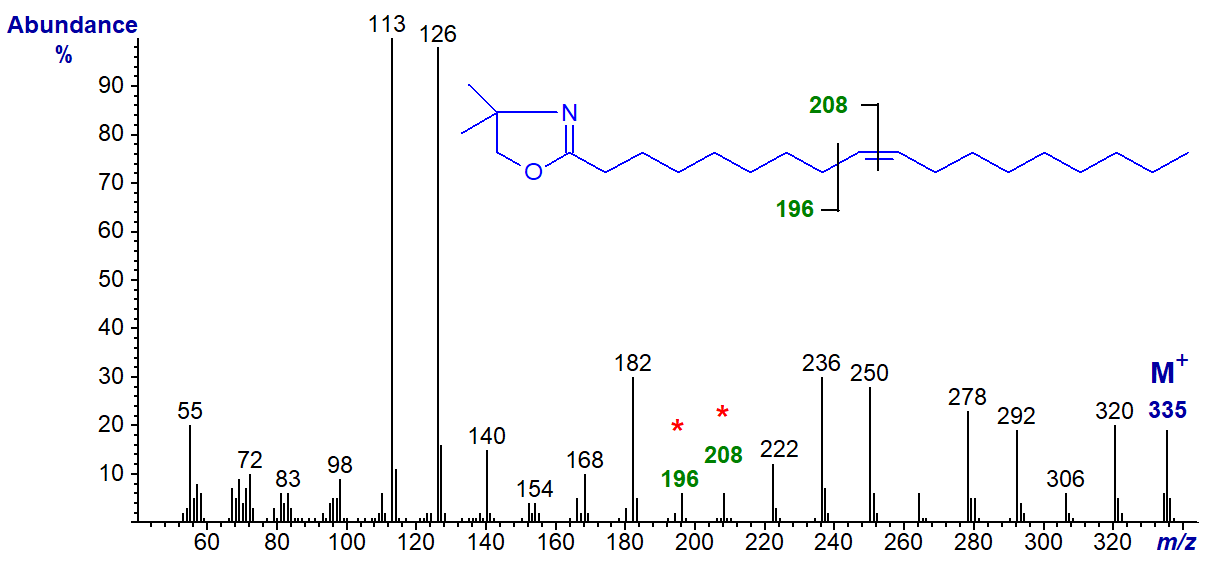
In this instance the ions at m/z = 113 and 126 confirm that we have indeed formed the DMOX derivative. Again, there is a clear molecular ion at m/z = 335, followed by gaps of 14 amu for the loss of each successive methylene group (m/z = 320, 306, 292, 278, etc.), until we find a gap of 12 amu which is indicative of the presence of the double bond, between m/z = 196 and 208. To locate this precisely, we must use the "12 mass rule", i.e.,
"if a there is an interval of 12 amu between the most intense peaks of clusters of ions containing n and n‑1 carbon atoms, there is a double bond between carbon n and n+1 in the molecule".
This may seem rather strange (it was first identified for pyrrolidide derivatives), but it works well in practice. Other functional groups, such as branch points or ring structures, are located as with 3-pyridylcarbinol esters. Problems of interpretation can arise when functional groups are near either end of the molecule, when it helps to have access to spectra of standards for comparison purposes as in the Archive pages of this website.
DMOX derivatives can be prepared simply by reacting the free fatty acid (or the methyl ester or even an intact lipid) with 2-amino-2-methyl-1-propanol in a micro-reaction vial at 180°C for 16 hours in a nitrogen atmosphere [8]. Practical details are available here..., where it is noted that the product must be stored under strictly anhydrous conditions otherwise partial hydrolysis can occur.
Pyrrolidides
Pyrrolidides are less used nowadays, although they still have their devotees, and they should not be forgotten. They are prepared from the methyl ester derivatives in a simple one-pot reaction with pyrrolidine in the presence of acetic acid at 100°C for 1 hour (see here...) In spite of the marked differences in structure, pyrrolidides have exactly the same molecular weight as the corresponding DMOX derivatives and they give very similar fragmentation patterns with electron impact ionization, although the diagnostic ions are often of lower abundance. They can be preferable to DMOX derivatives for fatty acids with near-terminal functional groups. Their GC properties are intermediate between those of 3-pyridylcarbinol esters and DMOX derivatives. As an example, the mass spectrum of the pyrrolidide of 14‑methylhexadecanoic acid is illustrated -
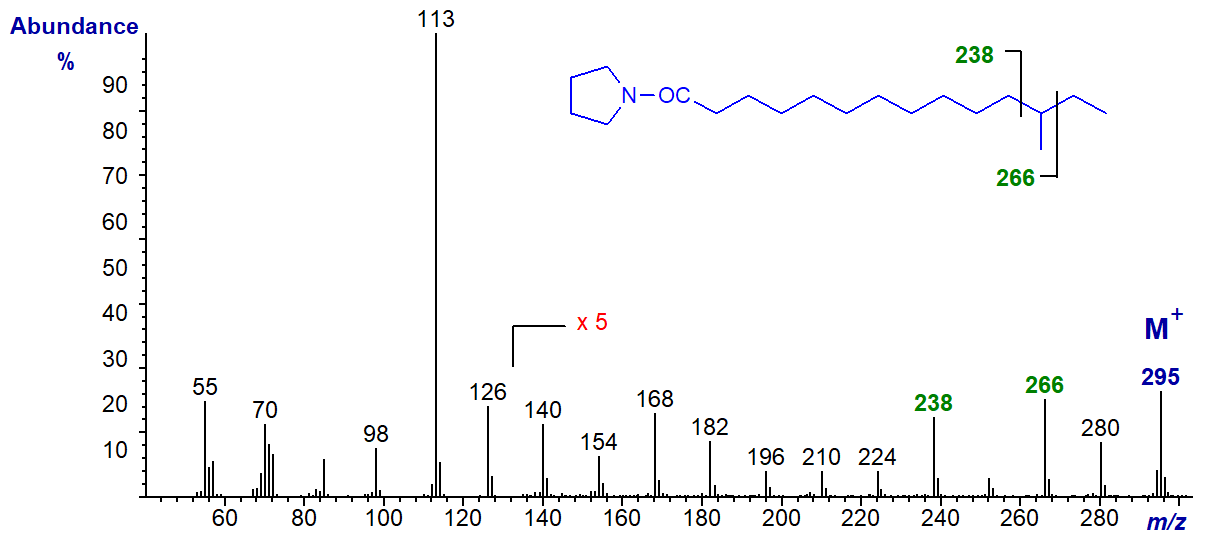
The ions for the carboxyl moiety at m/z = 113 and 126 are the same (other than intensity differences) as for the DMOX derivative for this fatty acid, as is the molecular ion at m/z = 323. The gap of 28 amu between m/z = 266 and 294 locates the methyl group, but there is no such diagnostic feature in the spectrum of the DMOX derivative of this fatty acid. It helps if the ions in the high mass region are magnified relative to the base ion to see this.
Which is Best?
How do we decide when to use 3-pyridylcarbinol esters and when DMOX or pyrrolidide derivatives for structural analysis of fatty acids? DMOX derivatives have excellent properties for gas chromatography, so they can be resolved easily on all the common polar stationary phases used in GC analysis. In my opinion, the mass spectral characteristics in the high mass range are not as good as with 3‑pyridylcarbinol esters in general, although DMOX derivatives are especially useful for some fatty acids with internal ring structures and cyclic and conjugated double bonds, but of less value when structural features are near the terminal carbon of a fatty acid. 3‑Pyridylcarbinol esters require much higher temperatures than the equivalent DMOX or methyl ester derivatives to elute them from GC columns, and at first, they could only be analysed on non-polar stationary phases. Later, we used the thermally stable polar BPX-70 and Supelcowax 10 columns with some success with 3‑pyridylcarbinol esters of fatty acids with up to 22 carbon atoms. I tend to use pyrrolidides for confirmatory purposes only, although they do have some distinctive GC properties.
Alternative GC-MS methods, such as that involving acetonitrile-chemical ionization, look interesting, as they may permit both the location of double bonds and their geometry (cis/trans), but they may be more limited in their potential range of application than the derivatives described above [9], and other 'soft' ionization methods are available for LC-MS [10].
With complicated samples containing larger numbers of different fatty acids, such as those of marine origin, it can be advisable to prepare simpler fractions by the methods described in my web page on this topic or in my book [4] prior to analysis by GC-MS.
Finally in answering the question of which is best, I prefer not to take a rigid stance. 3-Pyridylcarbinol esters were long my favourites for samples containing novel fatty acid structures, although I almost always prepared DMOX derivatives for confirmation or for rapid screening of straight-forward samples. Indeed, I often prepare and analyse methyl esters and pyrrolidides also, partly as this may permit better chromatographic resolution of some components and partly to obtain reference spectra. My firm belief is that the various types of derivative should be considered as complementary to each other and not simply as alternatives. That said, the simplicity of the method of preparing DMOX derivatives together with the lower elution times on GC-MS means that they are often the first choice for definitive characterization of fatty acids.
References

- Andersson, B.A. Mass spectrometry of fatty acid pyrrolidides. Prog. Chem. Fats other Lipids, 16, 279-308 (1978); DOI.
- Harvey, D.J. Mass spectrometry of picolinyl and other nitrogen-containing derivatives of fatty acids. In: Advances in Lipid Methodology - One, pp. 19-80 (W.W. Christie, ed., Oily Press, Dundee) (1992).
- Spitzer, V. Structure analysis of fatty acids by gas chromatography - low resolution electron impact mass spectrometry of their 4,4-dimethyloxazoline derivatives - a review. Prog. Lipid Res., 35, 387-408 (1997); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Harvey, D.J. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom., 9, 33-38 (1982); DOI.
- Balazy, M. and Nies, A.S. Characterization of epoxides of polyunsaturated fatty acids by mass spectrometry via 3-pyridinylmethyl esters. Biomed. Environ. Mass Spectrom., 18, 328-336 (1989); DOI.
- Destaillats, F. and Angers, P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc., 79, 253-256 (2002); DOI.
- Yu, Q.T., Liu, B.N., Zhang, J.Y. and Huang, Z.H. Location of methyl branches in fatty acids: Fatty acids in uropygial secretion of Shanghai ducks by gas chromatography-mass spectrometry of 4,4-dimethyloxazoline derivatives. Lipids, 23, 804-810 (1988); DOI.
- Brenna, J.T. Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent adduct chemical ionization. In: Lipid Analysis and Lipidomics: New Techniques and Applications. pp. 157-172 (ed: M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, USA) (2006).
- Chen, C., Li, R.J. and Wu, H. Recent progress in the analysis of unsaturated fatty acids in biological samples by chemical derivatization-based chromatography-mass spectrometry methods. J. Chromatogr. B, 1215, 123572 (2023); DOI.
A pdf ![]() file of the two parts of this beginner's
guide is available here..
file of the two parts of this beginner's
guide is available here..
| © Author: William W. Christie |  |
|
| Updated: February 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
