The Lipid Compositions of Animal Tissues
Comprehensive discussion of the enormous literature on compositions of animal, plant and microbial tissues would be a daunting task, and it is only possible here to summarize some of the more significant features of lipid composition, with some highly selective (and simplified) results from representative analyses. Further data are available for specific lipids in most of the web pages in this part of the website. There are short cuts to finding structural, compositional and biochemical information on individual lipid classes in this website from this link and at the end of all the web pages in this section as well as the search facility in the header.
One problem in comparing data from different sources is the method of presentation, which is often dependent on the nature of the analytical methods used. It can be easier technically to analyse phospholipids and glyco(sphingo)lipids following isolation as distinct groups, separately from the simple lipids, and often there is no attempt subsequently to integrate the data. To add to the confusion, results of analyses of simple lipids are often reported in terms of weight percent of each lipid class, since the data are acquired in this form, while those for phospholipids are most frequently recorded as molar percent, especially when phosphorus analysis is used as the means of quantification. Modern lipidomics analyses are based entirely on determination of molecular species of each lipid class by mass spectrometry, and the output is usually too complex to summarize in simple tables. In a single cell, it has been estimated that there are approximately 1,000 molecular species of lipids, while as many as 10,000 in total have been characterized from different tissues. For this reason, I have chosen to tabulate fatty acid positional distributions in glycerolipids to illustrate structural complexities, and although they are from publications that may appear relatively old, they are reliable.
1. Lipid Class Compositions
In animal tissues, the structural lipid components such as the phospholipids tend to be rather constant in composition under the normal physiological conditions so meaningful comparisons between different organs can often be made. On the other hand, the proportions of the simple lipids, especially the triacylglycerols, can vary greatly according to the dietary or physiological state of the animal, and this information is not always recorded in papers. Where possible, data are presented for the liver, because it contains all the important lipid classes and because of the central position of this organ in lipid metabolism. Fish lipids are discussed only briefly here, but Ackman has edited a two-volume work on lipids of marine origin [1].
Some representative data on the lipid composition of selected tissues from the rat, a common model animal for experimentation, are shown in Table 1. Those lipids listed are by far the most abundant in these tissues, and they are those most often seen in other organs, although other minor lipids can have biological relevance. Lipids present at relatively low concentrations only, such as most of the lysophospholipids, phosphatidic acid, polyphosphoinositides and ceramides, were not determined in these analyses, although they are of course of great metabolic importance. Nor were the diacyl, alkylacyl and alkenylacyl forms of the phospholipids distinguished. For comparison purposes, I have listed relative rather than absolute amounts.
Table 1. The composition of the lipid classes (weight % of the total) in rat heart, liver, erythrocytes and plasmaa. |
||||
| Lipid Class | Tissue | |||
|---|---|---|---|---|
| heart | liver | erythrocytes | plasma | |
| Cholesterol esters | trace | 2 | - | 16 |
| Triacylglycerols | 4 | 7 | - | 49 |
| Cholesterol | 4 | 5 | 30 | 6 |
| Diacylglycerols | 1 | - | trace | trace |
| Free fatty acids | - | trace | - | 2 |
| Cardiolipinb | 12 | 5 | - | - |
| Phosphatidylethanolamine | 33 | 20 | 21 | - |
| Phosphatidylinositol | 4 | 4 | 3 | - |
| Phosphatidylserine | - | - | 3 | - |
| Phosphatidylcholine | 39 | 55 | 32 | 24 |
| Sphingomyelin | 2 | 2 | 8 | 2 |
| Lysophosphatidylcholine | - | - | 1 | 1 |
| a Christie, W.W., J. Lipid Res., 26, 507-512 (1985);
DOI. b cerebrosides and phosphatidylglycerol were also in this fraction. |
||||
Cholesterol esters, triacylglycerols and free (unesterified) cholesterol tend to be the most abundant simple lipids. The choline-containing constituents, i.e., phosphatidylcholine and sphingomyelin, but predominantly the former, tend to be the main phospholipids (50 to 60% of the total), followed by phosphatidylethanolamine and then by phosphatidylinositol and phosphatidylserine. The results quoted for rat liver lipids are therefore typical of this and many other organs and possibly for other mammals. Cardiolipin is a major component of mitochondrial lipids and so is found in appreciable amounts in heart muscle, while phosphatidylglycerol is only present in significant amounts in lung surfactant. When substantial amounts of diacylglycerols, phosphatidic acid, lysophospholipids or free fatty acids, especially the last, are encountered in a sample, it is usually indicative of artefactual hydrolysis during storage or extraction of the tissues during processing or analysis.
 In erythrocytes, the lipids are mainly membrane constituents,
and the results of the analysis listed here indicate that only cholesterol and phospholipids are present as major components.
These data would be typical of many other animals, and any departure from this general pattern is symptomatic of an underlying metabolic
difference; for example, in ruminant erythrocytes, there is virtually no phosphatidylcholine, which is replaced entirely by sphingomyelin.
The fatty acid components of erythrocytes are often considered a better marker of long-term nutritional status than are those of the
plasma lipids.
In erythrocytes, the lipids are mainly membrane constituents,
and the results of the analysis listed here indicate that only cholesterol and phospholipids are present as major components.
These data would be typical of many other animals, and any departure from this general pattern is symptomatic of an underlying metabolic
difference; for example, in ruminant erythrocytes, there is virtually no phosphatidylcholine, which is replaced entirely by sphingomyelin.
The fatty acid components of erythrocytes are often considered a better marker of long-term nutritional status than are those of the
plasma lipids.
The compositions of the plasma lipids are of particular interest as they supply fatty acids to all tissues and are assumed to be in metabolic equilibrium with them, and of course, biopsy samples of plasma are relatively easy to obtain. Those lipids reported as plasma lipid constituents here are typical. Phosphatidylcholine is present as a higher proportion of the total phospholipids than in any other tissue, while cholesterol esters tend to be more abundant than in most organs (other than in steroidogenic tissues such as the adrenals). The proportion of triacylglycerols in the plasma in this instance was higher than normal because of the physiological state of the experimental animals. As lipids are transported in plasma as complexes with proteins, i.e., as lipoproteins, which render them compatible with their aqueous environment, it should be recognized that an analysis of the total lipids in plasma can give only part of the picture, and it may be necessary to determine the compositions of each of the lipoprotein fractions before definitive metabolic conclusions can be drawn.
Brain phospholipids are distinctive in that they tend to contain a higher proportion of phosphatidylserine (~10%) than other tissues, and much of the phosphatidylethanolamine especially is in plasmalogen form; sphingolipids make up a high proportion of the total complex lipids (see below).
There is no such thing as an average nucleated mammalian cell, but it is possible to give a list of the more abundant structural lipids (i.e., in membranes) together with a rough indication of the proportions in which they occur as in Table 2 (triacylglycerols and other simple lipids are storage as opposed to structural lipids so are not listed).
Table 2. Composition of structural lipids in an 'average' mammalian cell. |
|
| Lipid class | % |
| Phosphatidylcholine | 45‑55 |
| Phosphatidylethanolamine | 15-25 |
| Phosphatidylinositol | 10-15 |
| Phosphatidylserine | 5-10 |
| Phosphatidic acid | 1-2 |
| Sphingomyelin | 5-10 |
| Cardiolipin | 2-5 |
| Phosphatidylglycerol | <1 |
| Glycosphingolipids | 2-5 |
| Cholesterol | 10-20 |
| Adapted from Vance, J. Traffic, 16, 1-18 (2015); DOI. | |
All membranes (indeed each side of the bilayer) in a tissue can have distinctive compositions that are in some way related to their function. The results of some analyses of the phospholipids of the membranes of rat liver are recorded in Table 3. Naturally, the same phospholipids are present as is described above for the intact organ, but the relative proportions vary markedly, and for example, cardiolipin and sphingomyelin are much more abundant in mitochondria and plasma membrane, respectively, than in any of the other cellular membranes/organelles.
Table 3. The phospholipid composition of whole tissue and membrane preparations from rat liver (mol % lipid phosphorus). |
|||||
| Membrane | |||||
|---|---|---|---|---|---|
| Lipid class | Whole tissue |
Nuclei | Mitochondria | Microsomes | Plasma membrane |
| Cardiolipin | 5 | - | 15 | 2 | - |
| Phosphatidylethanolamine | 25 | 26 | 34 | 22 | 20 |
| Phosphatidylinositol | 7 | 4 | 7 | 8 | 7 |
| Phosphatidylserine | 3 | 6 | 1 | 4 | 4 |
| Phosphatidylcholine | 51 | 57 | 41 | 59 | 43 |
| Sphingomyelin | 4 | 6 | 2 | 4 | 23 |
| Lysophosphatidylcholine | 1 | - | 1 | 2 | 2 |
| [a] | [b] | [c] | [c] | [c] | |
| References: [a] Wuthier, R.E. J. Lipid Res., 7, 544-550 (1966); DOI. [b] Gurr, M.I. et al. Biochim. Biophys. Acta, 106, 357-370 (1965); DOI. [c] Colbeau, A. et al. Biochim. Biophys. Acta, 249, 462-492 (1971); DOI. | |||||
The data listed in Table 3 are incomplete as cholesterol is major constituent of some membranes that modifies their fluidity, and the proportion relative to the phospholipids should ideally be determined. In plasma membrane preparations from rat liver, the molar ratio of cholesterol to phospholipids is reported to be 0.76, while that in microsomes (endoplasmic reticulum) and mitochondria is 0.1 [2]. Data for minor phospholipid constituents such as polyphosphoinositides are only rarely tabulated. In addition, only a part of each phospholipid is the diacyl form, and in most tissues the phosphatidylethanolamine especially tends to contain an appreciable content of plasmalogens. On the other hand, phosphatidylcholine only occurs as plasmalogens in relatively high proportions in heart muscle, eye and reproductive tissues. With a few significant exceptions, the lipid compositions of equivalent membranes from different tissues and even from different animals are in general rather similar, probably because these membranes have common functional requirements (although the fatty acid compositions may differ depending on diet amongst other factors). In particular, the phospholipid composition of mitochondrial membranes is highly conserved from yeast to humans, with each lipid class being necessary for the assembly and activity of mitochondrial proteins, including those for oxidative phosphorylation (see our web page on cardiolipin).
For the reasons explained in the introduction to this document (and below), I have not been able to include data from modern lipidomics studies here, but I can recommend a review paper that reviews such studies as applied to cellular organelles [3].
2. Fatty Acid Compositions of Glycerolipids
The fatty acid composition of each lipid in a tissue is frequently distinctive and can vary markedly between animal species. It is obviously greatly, but not entirely, dependent on the nature of the diet of the animal concerned (although in many analytical studies, such details are not given). Kuksis [4] has reviewed the fatty acid compositions of animal glycerolipids. Data for the fatty acid compositions of the depot fats (mainly triacylglycerols), which are highly dependent upon diet, from several animals are listed in Table 4.
Table 4. The fatty acid compositions (mol % of the total) of the adipose tissue lipids of various animals. |
||||||
| Fatty acid | Animal | |||||
|---|---|---|---|---|---|---|
| Rat [a] | Pig [b] | Sheep [c] | Horse [a] | Herring [d] | Seal [d] | |
| 16:0 | 23 | 29 | 21 | 26 | 21 | 10 |
| 16:1 | 5 | 3 | 2 | 8 | 11 | 16 |
| 18:0 | 6 | 18 | 35 | 5 | - | - |
| 18:1 | 35 | 41 | 31 | 31 | 23 | 26 |
| 18:2 | 19 | 8 | 2 | 9 | 1 | 2 |
| 18:3 | 2 | - | - | 18 | ||
| 20:1 | 10 | 14 | ||||
| 20:5 | 9 | 7 | ||||
| 22:1 | 10 | 7 | ||||
| 22:6 | 5 | 8 | ||||
| References: [a] Brockerhoff, H. et al. Biochim. Biophys. Acta, 116, 67-72 (1966); DOI. [b] Christie, W.W. and Moore, J.H. Biochim. Biophys. Acta, 210, 46-56 (1970); DOI. [c] Christie, W.W. and Moore, J.H. J. Sci. Food Agric., 22, 120-124 (1971); DOI. [d] Brockerhoff, H. and Hoyle, R.J. Arch. Biochem. Biophys., 102, 452-455 (1963); DOI. | ||||||
In all, there are relatively high contents of 16:0 and 18:1 fatty acids, and species differences are most apparent in the relative concentrations of the saturated and polyunsaturated components. In rat, pig and horse, there are appreciable amounts of linoleic acid, but this is a minor component in sheep because it is subjected to microbial biohydrogenation in the rumen, and in herring and seal because it is present at low levels only in their food chain. Although the sheep and horse may consume a very similar diet, there are high levels of α-linolenic acid derived from the herbage in the lipids of the horse but not in those of the sheep, again because of biohydrogenation in the rumen of the latter. Fish such as the herring tend to contain relatively high amounts of C20 and C22 polyunsaturated fatty acids, derived mainly from the microflora and microfauna, which they consume. In turn, the composition of the depot fat of the seal reflects its diet of fish.
Each lipid class in a tissue has a characteristic fatty acid composition, probably related to its function but in a way that is still only partly understood, and some data for the main glycerolipids and the cholesterol esters of rat liver are listed in Table 5.
Table 5. The fatty acid compositions (mol % of the total) of the main glycerolipids and the cholesterol esters of rat liver. |
|||||||
| Fatty acid | Lipid class | ||||||
|---|---|---|---|---|---|---|---|
| TG [a] | PC [a] | PE [a] | PI [b] | PS [c] | Cardx [d] | CE [e] | |
| 16:0 | 27 | 14 | 18 | 7 | 4 | 7 | 24 |
| 16:1 | 4 | 1 | 8 | 3 | |||
| 18:0 | 7 | 34 | 37 | 40 | 47 | 4 | 20 |
| 18:1 | 27 | 10 | 8 | 3 | 3 | 20 | 15 |
| 18:2 | 12 | 12 | 5 | 3 | 2 | 59 | 16 |
| 20:4(n-6) | 1 | 20 | 23 | 40 | 25 | 2 | 14 |
| 22:6(n-3) | - | 4 | 7 | 2 | 16 | - | - |
| Abbreviations: TG, triacylglycerols; PC, phosphatidylcholine; PE, phosphatidylethanolamine;
PI, phosphatidylinositol; PS, phosphatidylserine; Card, cardiolipin; CE, cholesterol esters. x of the mitochondrial fraction References: [a] Wood, R. and Harlow, R.D. Arch. Biochem. Biophys., 131, 495-501 (1969); DOI. [b] Holub, B.J. and Kuksis, A. J. Lipid Res., 12, 699-705 (1971); DOI. [c] Holub, B.J. and Kuksis, A. Adv. Lipid Res., 16, 1-125 (1978). [d] Colbeau, A. et al. Biochim. Biophys. Acta, 249, 462-492 (1971); DOI. [e] Connellan, J.M. and Masters, C.J. Biochem. J., 94, 81-84 (1965); DOI. |
|||||||
In rat liver, only C16 and C18 fatty acids are found in significant amounts in the triacylglycerols, although most of the glycerophospholipids contain substantial proportions of the longer-chain polyunsaturated components. The phosphatidylcholine contains 50% of saturated fatty acids, but arachidonic acid constitutes 20% of the total, while the phosphatidylethanolamine has a similar proportion of saturated fatty acids, but somewhat less linoleic acid and correspondingly more of the C20 and C22 polyunsaturated fatty acids. Characteristically, high proportions of stearic and arachidonic acids are present in phosphatidylinositol, and this is true for phosphatidylserine except that the 22:6(n-3) fatty acid substitutes for part of the arachidonic acid. Cardiolipin, which is mainly from the mitochondrial compartment, differs markedly from all the other glycerophospholipids in that the single fatty acid, linoleic acid, comprises 60% and often more of the total. The composition of the cholesterol esters tends to resemble that of phosphatidylcholine, but there is somewhat less stearic and more palmitic acid in the former. In comparing the corresponding lipids of other animals, the same compositional trends in general are seen, although the absolute values may differ in part because of dietary factors. On the other hand, brain phospholipids are again distinctive in that they contain much higher proportions of docosahexaenoic acid especially in phosphatidylethanolamine.
3. Fatty Acid Positional Distributions within Glycerolipid Molecules
In addition to each glycerolipid class in a tissue having a characteristic fatty acid composition, each position of the glycerol moiety tends to have a unique fatty acid composition that is determined during the biosynthesis of a lipid by the specificities of various acyltransferases. Again, the positional distributions of fatty acids in particular lipid classes can vary markedly between tissues and animals and can have some metabolic importance. It should be noted that modern mass spectrometric methods cannot distinguish between positions sn-1 and sn-3 of triacylglycerols, i.e., it cannot determine the stereospecific distributions. Rather, they can only determine the composition of position sn-2 and the average of those of positions sn-1 and sn-3, which should be termed 'regiospecific' distributions. Stereospecific analysis of triacyl-sn-glycerols is technically daunting and requires a combination of chromatographic and enzymatic methodology. In contrast, mass spectrometric methods can now distinguish between the primary (sn-1) and secondary (sn-2) positions of glycerophospholipids.
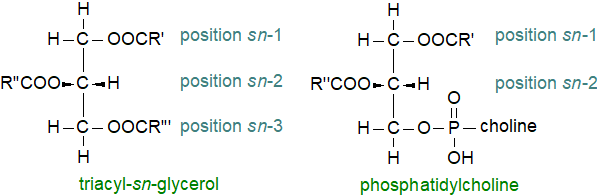 |
| Figure 1. Stereospecific distributions of fatty acids in glycerolipids. |
Data for triacylglycerols [5] and for glycerophospholipids [6] have been reviewed, and some results for the principal glycerolipids of rat liver taken from a single systematic analytical study [7] are listed in Table 6.
Table 6. Positional distributions of fatty acids in each position (sn-1, 2 or 3) of the principal glycerolipids of rat liver (results are expressed as mol % of the total in each position). |
|||||||
| Lipid class and position | |||||||
|---|---|---|---|---|---|---|---|
| Fatty acid | triacyl-sn-glycerol | phosphatidyl- choline |
phosphatidyl- ethanolamine |
||||
| sn-1 | sn-2 | sn-3 | sn-1 | sn-2 | sn-1 | sn-2 | |
| 16:0 | 57 | 13 | 12 | 23 | 6 | 25 | 11 |
| 16:1 | 4 | 3 | 4 | 1 | 1 | trace | trace |
| 18:0 | 9 | 4 | 8 | 65 | 4 | 65 | 8 |
| 18:1 | 22 | 58 | 61 | 7 | 13 | 8 | 8 |
| 18:2 | 4 | 19 | 11 | 1 | 23 | - | 10 |
| 20:4(n-6) | - | 1 | 1 | trace | 39 | - | 46 |
| 22:6(n-3) | - | 7 | - | 13 | |||
| Wood, R. and Harlow, R.D. Arch. Biochem. Biophys., 131, 495-501 (1969); DOI. | |||||||
In the triacylglycerols, the highest proportion of 16:0 is in position sn-1, but the other saturated fatty acid, 18:0, is distributed between positions sn-1 and sn-3 mainly. 16:1 is found in equal abundance in all three positions, but the C18 unsaturated fatty acids are distributed largely between positions sn-2 and sn-3. In phosphatidylcholine, much of the saturated fatty acids are in position sn-1, although a significant relative proportion of the 16:0 is found in position sn-2. Some of the 18:1 is in position sn-1, but virtually all the remaining di- and poly-unsaturated fatty acids are in position sn-2. Similar relative distributions are seen in phosphatidylethanolamine except that a slightly greater proportion of the saturated fatty acids are present in position sn-2.
1,2-Diacyl-sn-glycerols are a common intermediate in the biosynthesis of each of these lipid classes, but there is little correspondence in the compositions of this part of the molecules, when the phospholipids are compared with the triacylglycerols, and analyses of the molecular species distributions in the three classes of lipids indicated that there was little in common between the structures of the triacylglycerols and those of the phospholipids [6]. This suggests that either there is great selectivity for particular intermediate diacylglycerols for the synthesis of each lipid class, or more likely, that hydrolysis and re-acylation of lipids occurs by the Lands' cycle to give the final compositions.
 Much of the published work on the structures of animal triacylglycerols
has been concerned with those of adipose tissue, which tends to contain most of the body stores of fat as this single lipid class.
The results quoted here for rat liver triacylglycerols are like those obtained for adipose tissue for many animals.
In most instances, position sn-1 contains appreciable amounts of the saturated fatty acids, position sn-2 contains mainly
unsaturated and any shorter-chain fatty acids, and position sn-3 consists predominantly of unsaturated and longer-chain fatty acids.
The principal exception to this type of distribution was thought to be the triacylglycerols of the pig and its relatives, in which position
sn-2 is occupied largely by palmitic acid (70% or more) but more tissues containing triacylglycerols with a structure of this kind
are being revealed as research progresses.
In milk fats from all the higher mammals studied, more than half of their total content of palmitic acid is in position sn-2,
and the same is true of the triacylglycerols of lymph, plasma and adrenals in ruminant animals.
Another interesting feature of milk fats, especially those of ruminants, is that all the short-chain fatty acids (4:0 and 6:0 specifically)
are located exclusively in position sn-3.
Much of the published work on the structures of animal triacylglycerols
has been concerned with those of adipose tissue, which tends to contain most of the body stores of fat as this single lipid class.
The results quoted here for rat liver triacylglycerols are like those obtained for adipose tissue for many animals.
In most instances, position sn-1 contains appreciable amounts of the saturated fatty acids, position sn-2 contains mainly
unsaturated and any shorter-chain fatty acids, and position sn-3 consists predominantly of unsaturated and longer-chain fatty acids.
The principal exception to this type of distribution was thought to be the triacylglycerols of the pig and its relatives, in which position
sn-2 is occupied largely by palmitic acid (70% or more) but more tissues containing triacylglycerols with a structure of this kind
are being revealed as research progresses.
In milk fats from all the higher mammals studied, more than half of their total content of palmitic acid is in position sn-2,
and the same is true of the triacylglycerols of lymph, plasma and adrenals in ruminant animals.
Another interesting feature of milk fats, especially those of ruminants, is that all the short-chain fatty acids (4:0 and 6:0 specifically)
are located exclusively in position sn-3.
Many excellent analytical studies of the molecular species distributions in animal triacylglycerols have been published, but as the results are not easily summarized, readers are referred to a specialist review for detailed information [5]. Some comparative data and a more comprehensive discussion are available in our web page dealing with the compositions of triacylglycerols per se, while a further document in the series deals with the methodology for stereospecific analysis.
The positional distributions of fatty acids in each of the two most abundant phospholipids of rat liver, as listed in Table 6, are typical for these lipids in many other tissues and animals. In some tissues, the proportions of saturated fatty acids in position sn-2 can be somewhat higher, but polyunsaturated fatty acids rarely occur in significant amounts in position sn-1. Perhaps the best-known example of a glycerophospholipid containing mainly saturated fatty acids is the phosphatidylcholine of lung surfactant, of which up to 60% can be the dipalmitoyl species. The distribution of fatty acids in phosphatidylinositol follows the common pattern, and a high proportion has the 1-stearoyl,2-arachidonoyl structure. In the phospholipid analyses listed here, hydrolysis with the regiospecific phospholipase A2 from snake venom was used, but modern mass spectrometric methodology is now widely employed (although I am not aware of any objective comparison between the two approaches).
4. Sphingolipid Compositions
The sphingolipid components of many tissues and animals have been analysed, but those of bovine kidney have been studied in detail with respect both to the relative amounts in various regions of the organ and to the nature of the lipid and non-lipid moieties of the molecules [8,9]. Some data are listed in Table 7 for illustrative purposes.
Table 7. The sphingolipid composition of different bovine kidney regions (results expressed as mg lipid per gram dry tissue). |
|||
| Lipid class | Cortex | Medulla | Whole tissue |
|---|---|---|---|
| Ceramides | 1.0 | 0.7 | - |
| Glucosylceramides | 0.6 | 0.6 | 0.8 |
| Galactosylceramides | 0.2 | 1.3 | 0.4 |
| Di‑ & triglycosylceramides | 0.3 | 0.7 | 0.4 |
| Sulfatides | 0.1 | 0.9 | - |
| Sphingomyelins | 17.8 | 9.8 | 14.4 |
| Karlsson, A.A. In: Lipid Analysis in Oils and Fats, pp. 290-316 (edited by R.J. Hamilton, Blackie, London) (1998). | |||
Sphingomyelin is by far the most abundant sphingolipid, but significant amounts of ceramides and mono-, di- and triglycosylceramides and sulfatides are present also. The relative proportions of each vary appreciably at different sites in the kidney, presumably because of the differing functions of the membranes in each region.
The fatty acid composition of the sphingomyelin fraction of rat liver is listed in Table 8 [10]. In comparison to the glycerophospholipids, a very different range of fatty acids is present (see our Introduction to sphingolipids), and virtually no long-chain polyunsaturated fatty acids are esterified in sphingomyelin and other sphingolipids (although they are sometimes incorrectly identified as constituents), but C20 to C24 saturated and monoenoic fatty acids with odd or even chain-lengths are present as well as the normal C16 and C18 components. The same range of fatty acid constituents is seen in most other sphingolipids, and the bimodal chain-length distribution often causes individual sphingolipids separated by chromatographic means to appear as two adjacent bands. In addition, sphingoglycolipids frequently contain the same range of fatty acids to that listed but with a hydroxyl substituent in position 2.
Table 8. The fatty acid composition (weight % of the total) in the sphingomyelin of rat liver and the long-chain base composition (weight % of the total) of ceramide from human liver. |
|||
| Rat liver sphingomyelin [a] | Human liver ceramide [b] | ||
|---|---|---|---|
| Fatty acid | % | Long-chain base |
% |
| 16:0 | 22 | d16:0 | trace |
| 18:0 | 10 | d17:0 | trace |
| 18:1 | 4 | d16:1 | 5 |
| 18:2 | 2 | d18:0 | 2 |
| 20:0 | 2 | d17:1 | 3 |
| 22:0 | 14 | d18:1(i) a | 2 |
| 23:0 | 9 | d18:1 | 77 |
| 24:0 | 24 | d18:2 | 8 |
| 24:1 | 13 | d19:1(ai) | 3 |
| a abbreviations: i, iso-methyl branch; ai, anteiso-methyl branch. [a] Fex, G. Biochim. Biophys. Acta, Lipids, 231, 161-169 (1971); DOI. [b] Karlsson, A.A. In: Lipid Analysis in Oils and Fats, pp. 290-316 (ed. R.J. Hamilton, Blackie, London) (1998). |
|||
The long-chain base composition of ceramide, which is probably comparable to that of sphingomyelin, from human liver is also listed in Table 7. Sphingosine accounts for 77% of the total, but other sphingoid bases are present in small amounts. Relatively simple base compositions of this kind are found in the other sphingolipids of tissues from most simple-stomached animals. In ruminants, on the other hand, the long-chain base compositions can be much more complex, and it may include trihydroxy bases of which some are derived from herbage.
5. Lipidomic Analyses
As cautioned earlier, it is not easy to present the results of modern lipidomic analyses in a concise tabular fashion. Indeed, it concerns me that there is no easy method to exchange or compare such data between laboratories, although there is a campaign to change this. Modern mass spectrometry methods acquire data according to the molecular species structures of each lipid class and are generally presented in terms of the nature of the fatty acids in each. In the simplest form, these data can be tabulated as the sum of the properties of the fatty acid constituents, i.e., the total number of carbon atoms and double bonds in each, e.g., for a phospholipid class - 32:1, 36:2, 38:3, etc. - perhaps 20 to 30 in total, and each of these can contain several different components: 38:3 = 18:0-20:3, 20:0‑18:3, 20:1‑18:2, 18:1-20:2, etc. If positional distributions are taken into account, the number of different species can then run into hundreds, so it is technically easier to present data in graphical form rather than in tables. Hence the difficulty in comparing and presenting such data from different studies in general and especially here.
It would be remiss not to point readers to one such paper from the Lipid Maps consortium, which I suspect is destined to become a classic, i.e., an analysis of the lipids of macrophages, which shows what can be achieved when a large dedicated team tackles a problem with modern mass spectrometry [11]. Of course, since this was published, many equally authoritative studies have appeared in the scientific literature. I am not objective, but the book [12] is a useful guide to methodology.
References
- Ackman, R.G. (Editor) Marine Biogenic Lipids, Fats and Oils (two volumes) (CRC Press, Boca Raton, FL) (1990).
- Colbeau, A., Nachbaur, J. and Vignais, P.M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta, Biomembranes, 249, 462-492 (1971); DOI.
- Sarmento, M.J., Llorente, A., Petan, T., Khnykin, D., Popa, I., Perkovic, M.N., Konjevod, M. and Jaganjac, M. The expanding organelle lipidomes: current knowledge and challenges. Cell. Mol. Life Sci., 80, 237 (2023); DOI.
- Kuksis, A. Fatty acid composition of glycerolipids of animal tissues. In: Handbook of Lipid Research Vol. 1. Fatty Acids and Glycerides, pp. 381-442 (ed. A. Kuksis, Plenum Press, New York) (1978).
- Christie, W.W. The positional distribution of fatty acids in triglycerides. In: Analysis of Oils and Fats, pp. 313-339 (ed. R.J. Hamilton and J.B. Rossell, Elsevier Applied Science Publishers, London) (1986).
- Holub, B.J. and Kuksis, A. Metabolism of molecular species of diacylglycerophospholipids. Adv. Lipid Res., 16, 1-125 (1978).
- Wood, R. and Harlow, R.D. Structural studies of neutral glycerides and phosphoglycerides of rat liver. Arch. Biochem. Biophys., 131, 495-501 (1969); DOI.
- Karlsson, A.A. Analysis of intact polar lipids by high-pressure liquid chromatography-mass spectrometry/tandem mass spectrometry with use of thermospray or atmospheric pressure ionization. In: Lipid Analysis in Oils and Fats, pp. 290-316 (edited by R.J. Hamilton, Blackie, London) (1998).
- Karlsson, A.A., Arnoldsson, K.C., Westerdahl, G. and Odham, G. Common molecular species of glucosyl ceramides, lactosyl ceramides and sphingomyelins in bovine milk determined by high-performance liquid chromatography-mass spectrometry. Milchwissenschaft, 52, 554-559 (1997).
- Fex, G. Metabolism of phosphatidyl choline, phosphatidyl ethanolamine and sphingomyelin in regenerating rat liver. Biochim. Biophys. Acta, Lipids, 231, 161-169 (1971); DOI.
- Dennis, E.A. and 27 others. A mouse macrophage lipidome. J. Biol. Chem., 285, 39976-39985 (2010); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: February 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
