Mass Spectra of Some Miscellaneous Artefacts and Additives
 Extraneous
substances can be introduced into lipid extracts from innumerable sources.
Of course, some may be added deliberately - for example, 2,6‑di‑tert-butyl-4-methyl-phenol (BHT) to minimize autoxidation in
samples, while others may be introduced by accident.
Manufacturers of fine chemicals, like all humankind, are fallible,
and all laboratory reagents can on occasion contain impurities that may cause problems in analytical procedures. All solvents, including from time to time those grades that are nominally of high purity, can contain contaminants,
and as large volumes of solvent may be used to obtain small amounts of lipids, any such impurities can cause problems.
Plastic ware of all kinds (other than that made from Teflon™) can be especially troublesome and is best avoided
because plasticisers (diesters of phthalic acid usually) or elements of the polymers per se are very easily leached out.
These compounds tend to co-chromatograph with lipids, so they may spread confusion and obscure compounds of interest in chromatograms.
It is necessary to exercise vigilance to detect and eliminate these at an early stage.
Extraneous
substances can be introduced into lipid extracts from innumerable sources.
Of course, some may be added deliberately - for example, 2,6‑di‑tert-butyl-4-methyl-phenol (BHT) to minimize autoxidation in
samples, while others may be introduced by accident.
Manufacturers of fine chemicals, like all humankind, are fallible,
and all laboratory reagents can on occasion contain impurities that may cause problems in analytical procedures. All solvents, including from time to time those grades that are nominally of high purity, can contain contaminants,
and as large volumes of solvent may be used to obtain small amounts of lipids, any such impurities can cause problems.
Plastic ware of all kinds (other than that made from Teflon™) can be especially troublesome and is best avoided
because plasticisers (diesters of phthalic acid usually) or elements of the polymers per se are very easily leached out.
These compounds tend to co-chromatograph with lipids, so they may spread confusion and obscure compounds of interest in chromatograms.
It is necessary to exercise vigilance to detect and eliminate these at an early stage.
Further contaminants can arise from fingerprints and from a host of materials in everyday use in laboratories, including cosmetics, hair preparations, hand creams, soaps, polishes, the exhausts from vacuum pumps, lubricants and greases if they are used carelessly.
Some of those contaminants encountered in our work that have interfered with our mass spectrometry are described below. I have concentrated on the fingerprint spectra, not on the details of interpretation, as this is not usually relevant. You may also wish to consult our web page dealing with mass spectra of miscellaneous lipids, which contains spectra of some lipids that may be encountered infrequently but can crop up from time to time. There is a useful publication on this topic by Canez and Li (2024).
2,6-Di-tert-butyl-4-methyl-phenol (BHT)
BHT is widely used by lipid analysts to limit autoxidation in tissue extracts or in lipid preparations of various kinds. Its mass spectrum is -
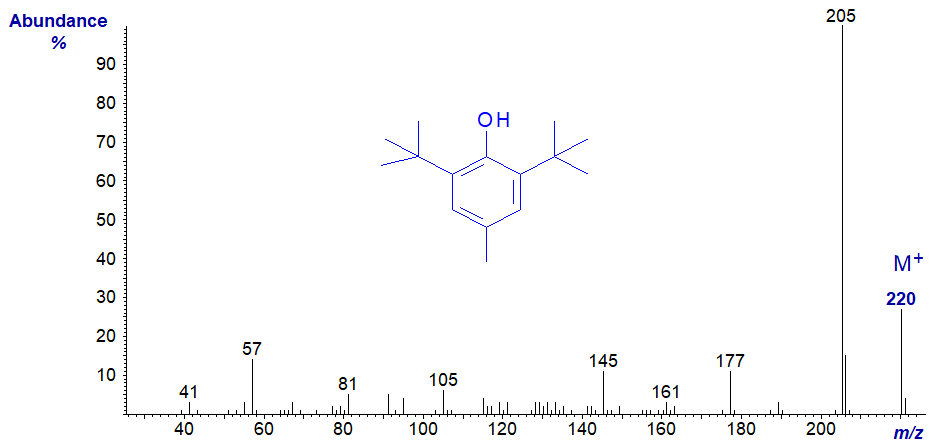
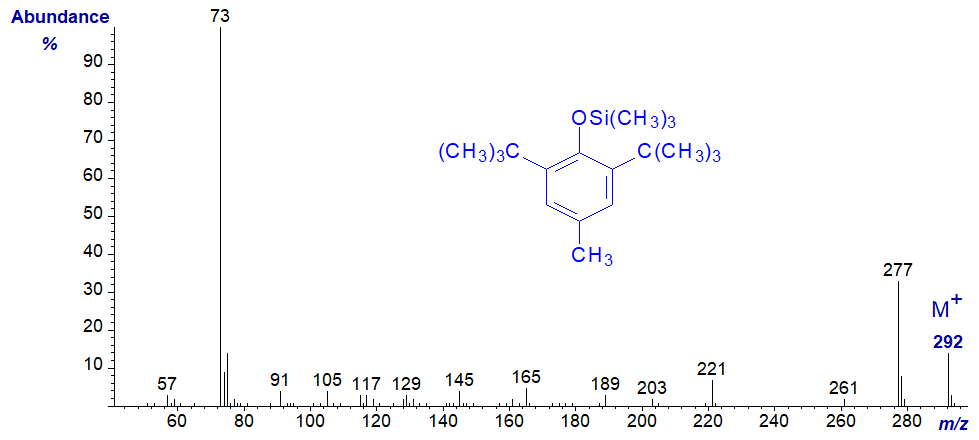
It tends to elute just before the methyl ester of 14:0 from most GC stationary phases, and it will inevitably be encountered in mass spectrometric analysis of fatty acid derivatives. An impurity lacking the 4-methyl group may be present in commercial products and has a very similar spectrum with the main ions 14 amu earlier (archived here..). Silylated BHT (next spectrum) may appear during the analysis of silylated lipid extracts, and it can cause confusion as it has a molecular weight in the same range as that of some fatty acid methyl esters.
Other Antioxidants
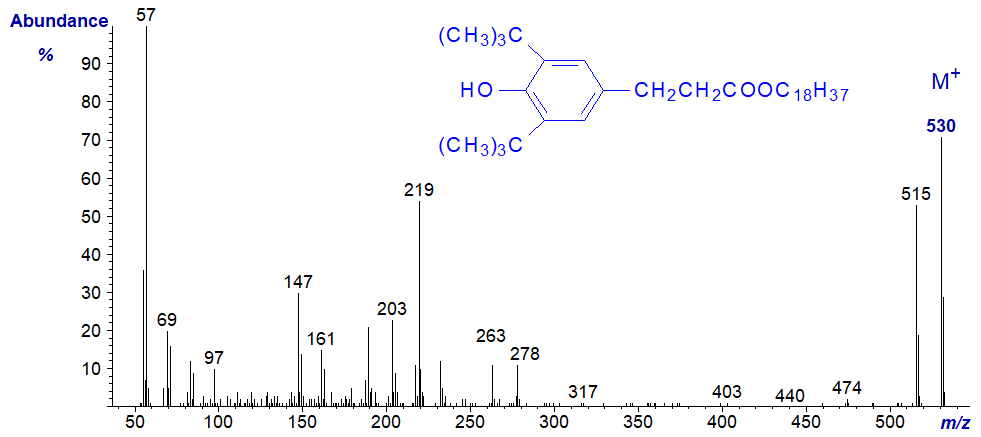
Innumerable other antioxidants may be found in lipid extracts, derived from plastics, packaging materials and others. Two produced by Ciba that we have found in our lipid preparations are illustrated below (with thanks to Claire Fernie). We have probably stumbled across more, but these two are listed in the commercial mass spectral library used in our laboratory so were easily identified.
Irganox 1076TM or octadecyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate -
Irgafos 168TM or tris(2,4-ditert-butylphenyl)phosphite
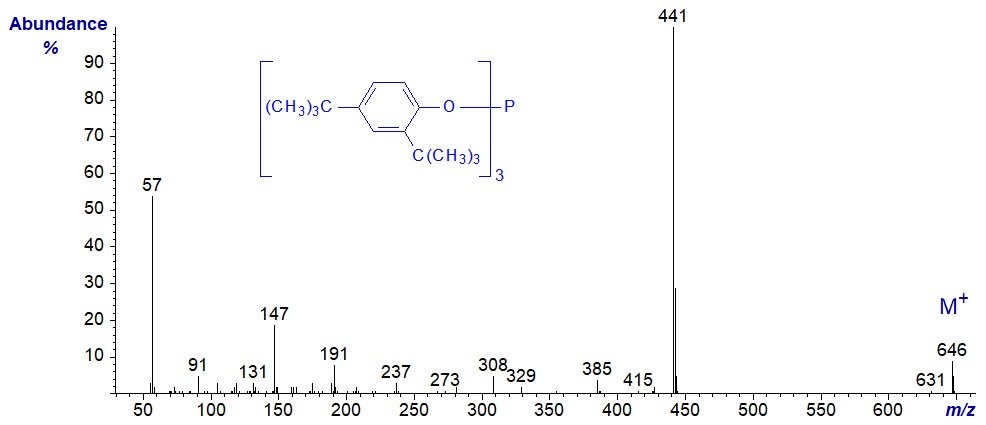
No interpretations of the spectra are offered here, but I can recommend a useful paper by Büchert et al. (1994).
Phthalates (Plasticisers)
Phthalate esters are widely used in the manufacturer of a wide range of plastics, and they are readily leached from these by contact with solvents, lipid extracts or even fresh tissues. They are especially troublesome in lipid analysis as they tend to elute with fatty acid derivatives from many of the common GC stationary phases. They are affected only slowly by transesterification reagents, although some basic reagents can give rise to sufficient partially or fully methylated phthalates to further confuse chromatograms (Shantha and Ackman, 1991). Unfortunately, they are not easily removed from lipid extracts or derivatives by adsorption or some other forms of chromatography. Two representative spectra are illustrated below -
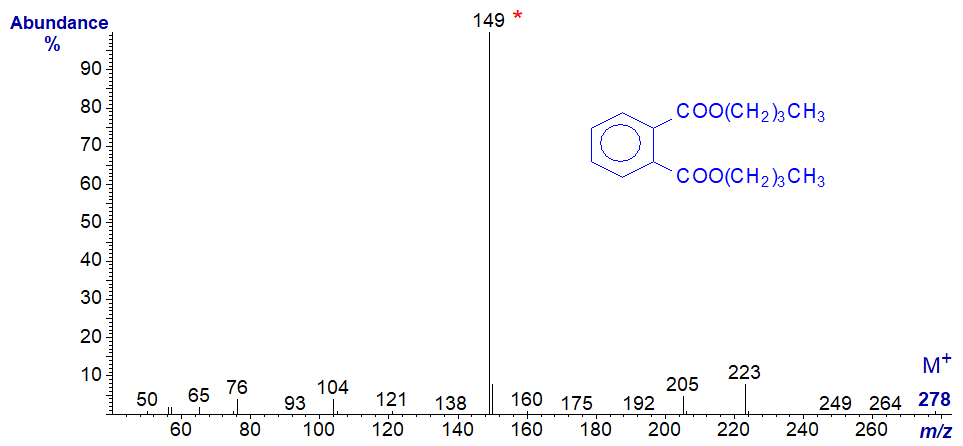
Dibutylphthalate -
Dioctylphthalate -
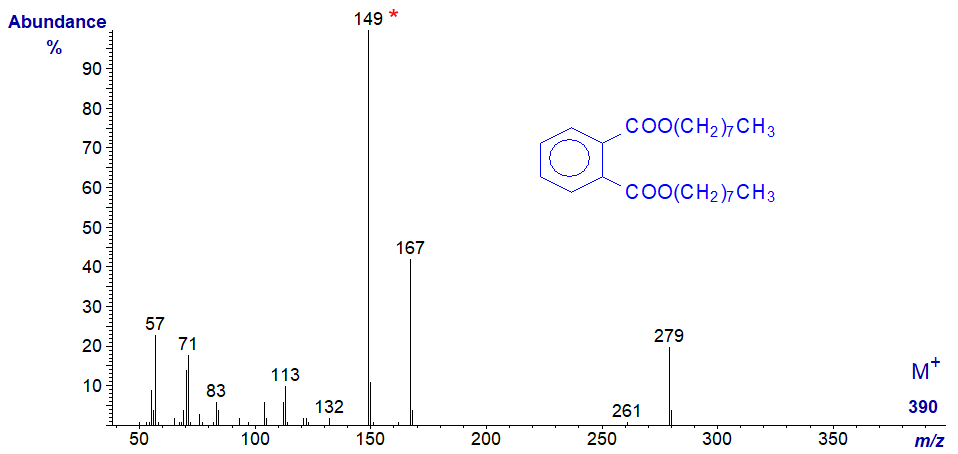
No interpretation of the spectra is offered here, other than that the key diagnostic feature in both is the base ion at m/z = 149.
Cyclic polymethylsiloxanes
Cyclic polymethylsiloxanes are easily leached from silicone rubbers, such as those found in composite Teflon™-lined caps for vials. They will appear in chromatograms if the septum is inserted into a GC sample vial the wrong way round, or if septa are damaged by over vigorous use. They appear as a long series of sharp peaks (20 or more components) that are regularly spaced in the chromatogram. Lower homologues are used as solvents in cosmetics, ointments and perfumes and can be introduced inadvertently into samples. We even found them in distilled water after a piece of plastic tubing was carelessly replaced by one of the wrong type. Some representative spectra are illustrated below.
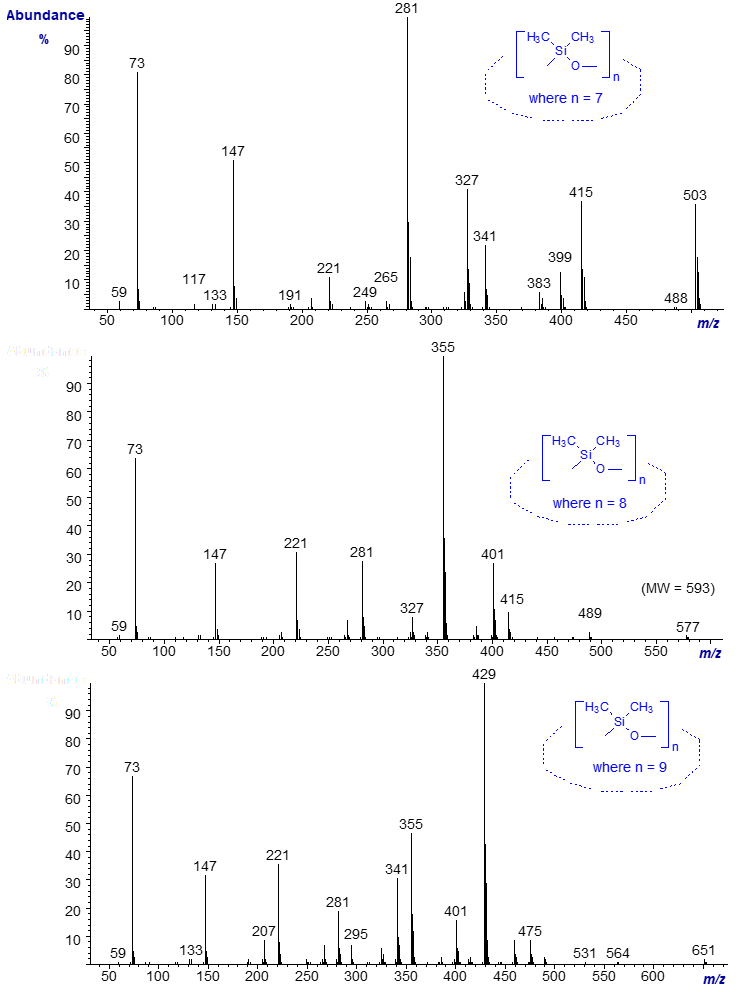
The molecular ions are not detected, and the ion with the highest m/z value in each spectrum represents the loss of a methyl group ([M‑15]+). Each homologue differs in molecular weight by the equivalent of 74 amu (one dimethyl siloxane unit). Again, no detailed interpretation is offered, but the key diagnostic features are a series of ions spaced approximately 70 amu apart, especially in the lower mass range, e.g., at m/z = 73, 147, 221, 295, 355, etc. The base ions in each of the three spectra illustrated are 74 amu apart, i.e., at m/z = 281, 355 and 429, and this series continues with further homologues in each direction. Further spectra are available in the Archive page.
References
- Canez, C.R. and Li, L. Studies of labware contamination during lipid extraction in mass spectrometry-based lipidome analysis. Anal. Chem., 96, 3544-3552 (2024); DOI.
- Büchert, T., Gruner, A. and Palibroda, N. Rapid analysis of polymer homologues and additives with SFE/SFC-MS coupling. Packaging Technol. Sci., 7, 139-154 (1994); DOI.
- Shantha, N.C. and Ackman, R.G. Behaviour of a common phthalate plasticizer (dioctyl phthalate) during the alkali- and/or acid-catalysed steps in an AOCS method for the preparation of methyl esters. J. Chromatogr. A, 587, 263-267 (1991); DOI.
| © Author: William W. Christie |  |
|
| Updated: March 27th, 2024 | Contact/credits/disclaimer | |
