Hydroxyeicosatetraenoic Acids and Related
Mono-Oxygenated Oxylipins
The oxygenated metabolites or oxylipins derived from arachidonic and related fatty acids are produced through a series of complex, interrelated biosynthetic pathways often termed the 'eicosanoid cascade'. Here, the linear hydroxyeicosatetraenes and related mono-oxygenated metabolites are described, together with octadecanoids produced from linoleate and comparable oxylipins from eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids. While these are relatively simple in structure, they are precursors for families of more complex molecules, such as the leukotrienes and lipoxins and the protectins, resolvins and maresins (or 'specialized pro-resolving mediators'). The two main enzymatic pathways for production of these eicosanoids utilize lipoxygenases (LOXs) and oxidases of the cytochrome P-450 family, although some are produced as minor by-products of cyclooxygenases or when cyclooxygenase 2 is inhibited by aspirin, as discussed in our web page on prostanoids, which have ring structures in the centre of the molecule and are discussed on their own web page. Hydroperoxides can also be formed non-enzymatically (autoxidation) as discussed in our web page dealing with isoprostanes.
1. Lipoxygenases and Hydroxyeicosatetraenoic Acids
Lipoxygenases are a family of enzymes that are characterized as non-heme iron proteins or dioxygenases, which catalyse the abstraction of hydrogen atoms from bis-allylic positions (1Z,4Z-pentadiene groups) of polyunsaturated fatty acids followed by stereospecific addition of dioxygen to generate hydroperoxides. They occur widely in plants, fungi, a few prokaryotes (cyanobacteria and proteobacteria) and animals, but not in the archaea and most insects. The plant lipoxygenases have distinctive substrates and products, and they are described in our web page dealing with plant oxylipins rather than here, although interesting parallels can be drawn with the mechanisms and functions of the animal enzymes.
 Animal
lipoxygenases that utilize arachidonic acid as substrate are of great biological and medical relevance
because their products serve in signalling or in inducing structural or metabolic changes in the cell.
They react with arachidonic acid per se to produce hydroperoxides in characteristic positions and thence by downstream processing
the plethora of eicosanoids, each with their own functions, which are described in this and other web pages and include lipoxins, trioxilins,
leukotrienes, hepoxilins, eoxins and specialized pro-resolving mediators.
Only the primary lipoxygenase products are discussed in this web page.
These enzymes can react to some extent directly with phospholipids in membranes to produce hydroperoxides and further metabolites that
perturb the membranes to induce structural changes in the cell as in the maturation of red blood cells.
Beyond their role in oxylipin production, lipoxygenases and lipid hydroperoxides act more generally in cellular redox homeostasis
and can stimulate the formation of secondary products, which, for example, can attack low-density lipoproteins directly with major implications
for the onset of atherosclerosis.
Animal
lipoxygenases that utilize arachidonic acid as substrate are of great biological and medical relevance
because their products serve in signalling or in inducing structural or metabolic changes in the cell.
They react with arachidonic acid per se to produce hydroperoxides in characteristic positions and thence by downstream processing
the plethora of eicosanoids, each with their own functions, which are described in this and other web pages and include lipoxins, trioxilins,
leukotrienes, hepoxilins, eoxins and specialized pro-resolving mediators.
Only the primary lipoxygenase products are discussed in this web page.
These enzymes can react to some extent directly with phospholipids in membranes to produce hydroperoxides and further metabolites that
perturb the membranes to induce structural changes in the cell as in the maturation of red blood cells.
Beyond their role in oxylipin production, lipoxygenases and lipid hydroperoxides act more generally in cellular redox homeostasis
and can stimulate the formation of secondary products, which, for example, can attack low-density lipoproteins directly with major implications
for the onset of atherosclerosis.
General mechanisms: The nomenclature of animal lipoxygenases is based on the specificity of the enzymes with respect to the products of the reaction with arachidonate (not the initial point of hydrogen abstraction); for example, 12-LOX oxygenates arachidonic acid at carbon-12, and the stereochemistry of the reaction can be specified, e.g., 12R-LOX or 12S-LOX, although most enzymically produced hydroperoxides have the S‑configuration. Where more than one enzyme has the same specificity, it may be named after the tissue in which it is found, and there are platelet, leukocyte and epidermal types of 12‑LOX.
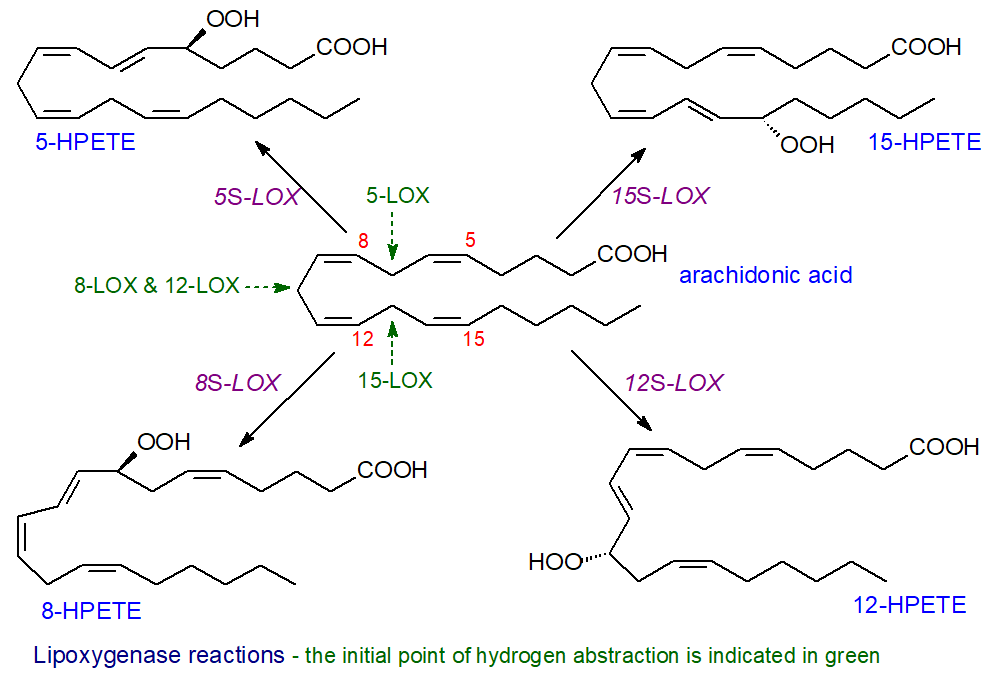
As the research in this area has developed, this simplistic nomenclature has become confusing as some enzymes can oxygenate more than one position and this can vary with the chain-length of the polyunsaturated substrate and the positions of the double bonds. Enzymes that act upon four different positions in arachidonic acid occur in animal tissues, i.e., 5‑LOX, 8-LOX, 12-LOX and 15‑LOX, although some of these have dual specificities, while many iso-forms exist depending on species. In humans, there are now considered to be six main lipoxygenase family members (5‑LOX, 12‑LOX, 12/15‑LOX (15‑LOX type 1), 15‑LOX type 2, 12R‑LOX and epidermal LOX (eLOX-3)) with seven in mice. Orthologues of the same gene can have different positional selectivities in different species, and mice do not express a distinct 15‑LOX but rather a leukocyte-derived 12-LOX with some 15‑LOX activity, so it can be difficult to extrapolate from animal experiments to human conditions; the human enzymes only are discussed at length here.
Each of the lipoxygenase proteins in animal tissues has a single polypeptide chain with a molecular mass of 75-80 kDa. They have an N‑terminal 'β‑barrel' or 'PLAT' domain, which is believed to acquire the substrate, and a larger α-helical catalytic domain containing a single atom of non-heme iron, which is bound to four conserved histidine residues and to the carboxyl group of a conserved isoleucine at the C-terminus of the protein. The PLAT domain anchors the otherwise cytosolic protein to membranes in response to intracellular calcium levels. For catalysis to occur, the iron component of the enzymes must be oxidized to the ferric state.
All the enzymes appear to include the fatty acid substrate within a tight channel with smaller channels that direct molecular oxygen toward the selected carbon to facilitate the formation of hydroperoxy-eicosatetraenes (HPETEs). In other words, the regiospecificity is regulated by the orientation and depth of substrate entry into the catalytic site, while stereospecificity is controlled by switching the position of oxygenation on the reacting pentadiene of the substrate at a single enzyme site, which is conserved as an alanine residue in S‑lipoxygenases and a glycine residue in the rarer R‑lipoxygenases. There is evidence that two amino acids opposite the catalytic iron ion determine the orientation of the substrate for entry into the enzyme channel. With 5‑LOX and 8‑LOX, the carboxyl group of arachidonic acid enters the catalytic site first, while with 12-LOX and 15-LOX, the ω‑terminus enters the site and assists the reaction. The N-terminal domains serve in membrane binding and regulation and are not utilized for catalysis.
Lipoxygenase action is believed to proceed in four steps - hydrogen abstraction (1), radical rearrangement (2), oxygen insertion (3) and peroxy radical reduction (4), all occurring under strict steric control, as illustrated.
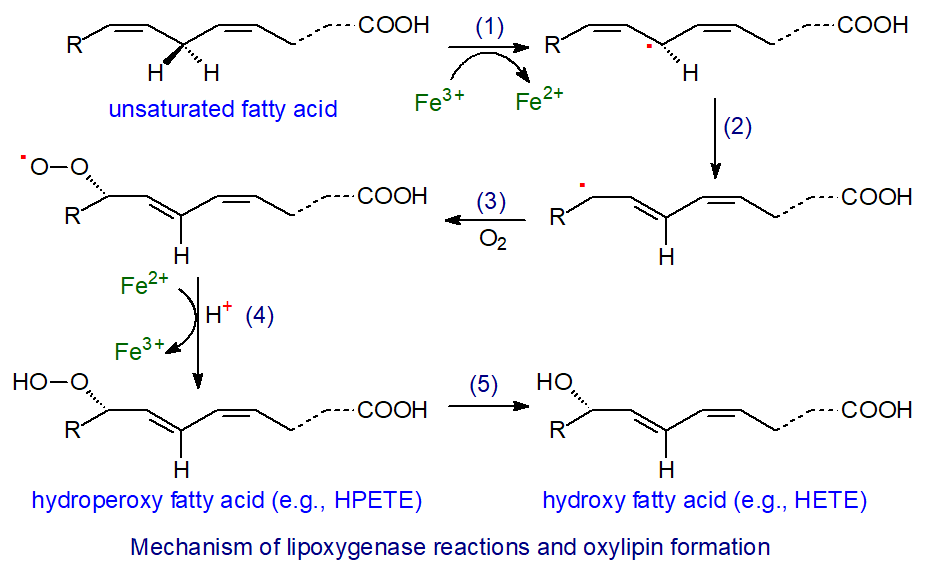
For example, in the action of 5-LOX, the first and rate-limiting step is the abstraction of a hydrogen atom from carbon 7 of arachidonic acid by non-heme ferric iron (Fe(III)), involving a proton-coupled electron transfer in which the electron is transferred directly to the iron and the proton is acquired simultaneously by the hydroxide ligand in a concerted mechanism to produce a substrate radical, while the iron atom is reduced to the ferrous form (Fe(II)). The cis-double bond in position 5 migrates to position 6 to form a more stable conjugated diene with a change to the trans-configuration before dioxygen is introduced opposite to the removed hydrogen (antarafacially) to generate a lipid peroxyl radical. Finally, the lipid peroxyl radical is reduced by Fe(II) and protonated to form a lipid hydroperoxide in another concerted reaction. In the process, the iron atom is re-oxidized to its ferric form for another round of catalysis. The oxylipin produced in the reaction illustrated is 5S‑hydroperoxy-6t,8c,11c,14c-eicosatetraenoic acid (5‑HPETE).
HPETE in general have a short half-life and are rapidly metabolized to hydroxy-eicosatetraenes (HETE) with the same stereochemistry, often via reduction by the abundant and ubiquitous glutathione peroxidases (step 5). While they are primarily intermediates in the biosynthesis of other eicosanoids, HPETE can have some more direct influence on tissue metabolism.
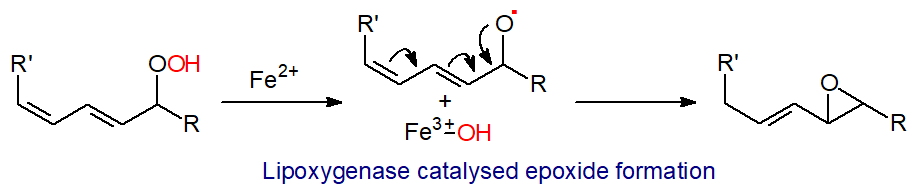
Alternatively, isomerization reactions of hydroperoxides can occur to produce leukotrienes and lipoxins via epoxy intermediates. Simplistically, the Fe2+ in the lipoxygenase cleaves the O-O bond in the hydroperoxide with transfer of the hydroxyl group to form Fe3+‑OH, before the residual alkoxyl radical is cyclized to form an epoxy fatty acid.
Enzyme specificities: 5-LOX (ALOX5) is found only in cells derived from bone marrow (leukocytes, macrophages, etc.), and it is of particular interest in that the products are the primary precursors for the leukotrienes and lipoxins and for resolvins. It is a cytosolic protein when intracellular calcium levels are low, but it becomes associated with the nuclear membrane when they are high or after phosphorylation. In contrast to other lipoxygenases, it requires the presence of a particular activator protein on the perinuclear membrane, lipoxygenase-activating protein (FLAP), which facilitates the transfer of arachidonic acid to the catalytic site on 5-LOX and is believed to accomplish the functional coupling of phospholipase A2 (cPLA2) to 5-LOX at the membrane. It is noteworthy that both cPLA2 and 5‑LOX are Ca2+‑dependent. 5-LOX and related enzymes are regulated by several factors that include the concentration and availability of the substrates, the redox state, intracellular Ca2+ concentrations, and phosphorylation-dephosphorylation by means of various protein kinases.
 8-, 12-
and 15-LOX operate in the same way to give analogous products and associate with membranes in a calcium-dependent process,
although they do not require accessory proteins.
15‑LOX exists in two forms, usually with one having a broader selectivity that depends on the animal
source, hence the superfluity of names, and it is expressed primarily in reticulocytes and macrophages on stimulation by interleukins 4 and 13
(12S-LOX of mice, leukocyte-type 12S-LOX of rabbits, reticulocyte-type 15S-LOX of rabbits and human
reticulocyte-type 15S-LOX).
8-, 12-
and 15-LOX operate in the same way to give analogous products and associate with membranes in a calcium-dependent process,
although they do not require accessory proteins.
15‑LOX exists in two forms, usually with one having a broader selectivity that depends on the animal
source, hence the superfluity of names, and it is expressed primarily in reticulocytes and macrophages on stimulation by interleukins 4 and 13
(12S-LOX of mice, leukocyte-type 12S-LOX of rabbits, reticulocyte-type 15S-LOX of rabbits and human
reticulocyte-type 15S-LOX).
The main product of 15-LOX in in humans is 15S-HPETE, and this form of the enzyme is then best termed 15‑LOX‑1 (alternatively, ALOX15 or 12/15‑LOX), but it can produce some 12‑HETE, 8,15‑diHETE and eoxin A4 from arachidonic acid. It can oxidize linoleate to 13‑hydroperoxy-octadecadienoate (and in part to the 9-isomer), as well as oxidizing α‑linolenic, γ‑linolenic, eicosapentaenoic and docosahexaenoic (DHA) acids. 15S‑HPETE is induced by the action of cytokines and is the precursor of the pro-resolution lipoxins, while with DHA, 15-LOX produces 17S‑HPDHA, a precursor of resolvins and protectins. Uniquely, 15‑LOX‑1 synthesises both pro- and anti-inflammatory molecules, and molecular genetics studies show that this feature is seen only in higher ranked primates and not in mammals ranked in evolution lower than gibbons, where the enzyme only produces the 12‑isomer with arachidonate.
The second human arachidonate 15‑lipoxygenase has 40% homology with the first and is termed 15‑LOX‑2 (or ALOX15B). It produces 15-HETE exclusively from arachidonate and is expressed constitutively in macrophages, where it has been associated with cellular cholesterol homeostasis and is induced by hypoxia, and in the prostate gland, lung, skin and cornea. Like 15-LOX-1, it differs from the other lipoxygenases in that it can utilize most polyunsaturated fatty acids as substrates, both in unesterified form and bound to intact lipids, including phospholipids and cholesterol esters in membranes and lipoproteins. Hence the interest in the enzyme in autophagy, membrane disruption and disease states (asthma, psoriasis and atherosclerosis). Mouse skin produces a lipoxygenase (8‑LOX) that is structurally related to 15-LOX-2, but generates 8S-HETE and 8S,15S-diHPETE from arachidonic acid. Some 15R-HETE is produced by the action of COX-2 and aspirin.
12S-LOX (ALOX12) from human platelets and leukocytes was one of the first lipoxygenases to be characterized, but a rather different enzyme is present in the epidermis. Although lipoxygenase metabolites generally have a hydroperoxide moiety in the S‑configuration, lipoxygenases in mammalian skin can produce the R‑form. Indeed, 12R‑HETE was first characterized as a component of psoriatic lesions. One of the enzymes responsible is a second form of the human 15‑lipoxygenase (15-LOX-2), but there is also a 12R-LOX (ALOX12B) with distinctive functions in keratinocytes and certain other tissues in relation to linoleate metabolism and the formation of structural ceramides in the corneocyte envelope. eLOX3 in skin is a hydroperoxide isomerase (lipohydroperoxidase) and transforms hydroperoxides to epoxy-alcohols and ketones (enzymes related to this are common in aquatic invertebrates).
Hydroxy fatty acids produced by lipoxygenases can be further oxidized to their keto analogues (cf., 5-oxo-eicosatetraenoic acid below) or to dihydroxy derivatives that include the leukotrienes discussed in a separate web page; some form glutathione conjugates.
2. Cytochrome P450 Oxidases and Hydroxy/Epoxy-Eicosatetraenoic Acids
Arachidonic acid can be oxidized by several cytochrome P450 mixed-function oxidases to produce various HETE isomers (the name was coined to describe the first such enzyme to be characterized and was based on an unusual absorbance peak at 450 nm from its carbon monoxide-bound form). These enzymes are a superfamily of membrane-bound hemoproteins that catalyse the scission of the dioxygen bond in molecular oxygen and transfer a single atomic oxygen to a substrate carbon atom, i.e., they are monooxygenases (with the release of the other oxygen atom as water). The result is the introduction of either a hydroxyl or an epoxyl group into the molecule. The reaction is NADPH-dependent, requiring transfer of electrons from NADPH to the P450 heme iron (lipoxygenases use non-heme iron) for which a membrane-bound enzyme partner, NADPH-cytochrome P450 reductase, is essential in the endoplasmic reticulum (or functionally related enzymes in mitochondria).

Cytochrome P450 oxidases are found in all mammalian cell types and indeed appear to be ubiquitous in all living organisms, although the number and distribution of each form of the enzymes are characteristic both for cell type and species. They are located in the endoplasmic reticulum with a limited expression in mitochondria (and perhaps plasma membrane and nucleus) and predominantly in the liver but with significant levels in some other extrahepatic tissues, including brain, kidney and lung. As well as generating HETE isomers, enzymes of this kind are part of the eicosanoid cascade in the metabolism of prostanoids, and they are utilized in cholesterol and steroid metabolism as well as detoxification of lipophilic xenobiotics, including drugs and chemical carcinogens. Their nomenclature starts with the root 'CYP', followed by a number allocated to the family, a letter for subfamily and a gene-identifying number for isoforms.
CYP450s have two key domains: a β-sheet-rich N-terminal domain and a larger helix-rich C-terminal catalytic domain. Those enzymes in the endoplasmic reticulum have a transmembrane helix in the N-terminal domain that is required for membrane anchoring, but this feature is not present in mitochondrial CYPs, which rely upon hydrophobic regions on the surface to bind to membranes. The catalytic domain contains the heme prosthetic group in a deep cavity, and variability in the structure of this site in each form explains the flexibility for substrates and products. Access channels permit entry of substrates, and exit channels allow egress of the product. Three types of reaction have been observed in animal cells that lead to the formation of three families of eicosanoids, all requiring unesterified arachidonic acid as substrate, although appreciable amounts of the products are eventually esterified.
Mid-chain HETE: Synthesis of mid-chain HETEs is accomplished by CYP1B1, CYP4A or CYP2B enzymes in reactions at bis‑allylic centres and is lipoxygenase-like in the nature of the ultimate HETE products, although not via hydroperoxy intermediates. Thus, these microsomal cytochrome P450 oxidases can react with arachidonic acid to produce six regioisomeric cis,trans-conjugated dienols, i.e., with the hydroxyl group in positions 5, 8, 9, 11, 12 or 15. The mechanism is believed to involve bis-allylic oxidations at either carbon-7, 10 or 13, followed by acid-catalysed rearrangement to the cis,trans-dienol (two of the possible products are illustrated). 12R-HETE as opposed to the 12S-isomer is a major product of the reaction, and this was at one time though to be a distinguishing feature, but some other lipoxygenases are now known to produce the former enantiomer.
Omega-hydroxylated HETE: Secondly, there are ω- and (ω-1)-hydroxylases that introduce a hydroxyl group into positions 20 and 19, respectively, of arachidonic acid mainly, although other enzymes can react at positions 16, 17 and 18. The reaction was first observed with medium-chain saturated fatty acids, such as lauric (12:0), where it may play a part in oxidative catabolism. Some isoenzymes act only upon laurate, others with arachidonate, and some will utilize both fatty acids as substrates. In humans, the iso-forms CYP4A and CYP4F are the main enzymes for ω‑hydroxylation of polyunsaturated fatty acids, including both arachidonic and eicosapentaenoic acids, while the CYP1A1, CYP2C19, and CYP2E1 forms perform (ω‑1)-hydroxylations. Both R- and S-forms of the sub-terminal HETE with differing activities can be produced.
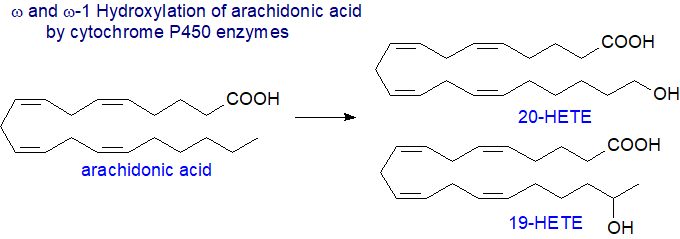
20-HETE is metabolized by cyclooxygenases into a hydroxy analogue of prostaglandin H2 (20-OH PGH2), a vasoconstrictor that is further converted by isomerases into 20-OH PGE2 and 20-OH PGI2 (vasodilator/diuretic metabolites) and 20‑OH thromboxane A2 and 20-OH PGF2α (vasoconstrictor-antidiuretic metabolites). Some CYP450 enzymes can introduce hydroxyl groups into positions 2 and 3 of fatty acids, while others can catalyse decarboxylation or form terminal alkenes with biotechnological potential.
Epoxyeicosatrienoic acids: In the third series of reactions of P450 arachidonic acid monooxygenases epoxytrienoic acids (‘EET’) are formed from arachidonic acid, i.e., four cis-epoxyeicosatrienoic acids (14,15-, 11,12-, 8,9- and 5,6-EETs). Apart from the 5,6-isomer, they are relatively stable molecules.
Several iso-enzymes of the cytochrome P450 epoxygenase exist, with CYP2C and CYP2J as the most active, and they can produce all four EET regioisomers, although one isomer tends to predominate in each tissue usually. For example, epoxygenases that produce 14,15-EET as the main isomer synthesise a significant amount of 11,12‑EET and a little 8,9-EET. The epoxygenase attaches an oxygen atom to one of the carbons of a double bond of arachidonic acid, and as the epoxide forms, the double bond is reduced. The enzymes are located both in the cytosol and the endoplasmic reticulum of endothelial cells, and they make use of arachidonic acid that is hydrolysed from phospholipids when the Ca2+-dependent phospholipase A2 is activated and translocated from the cytosol to intracellular membranes.
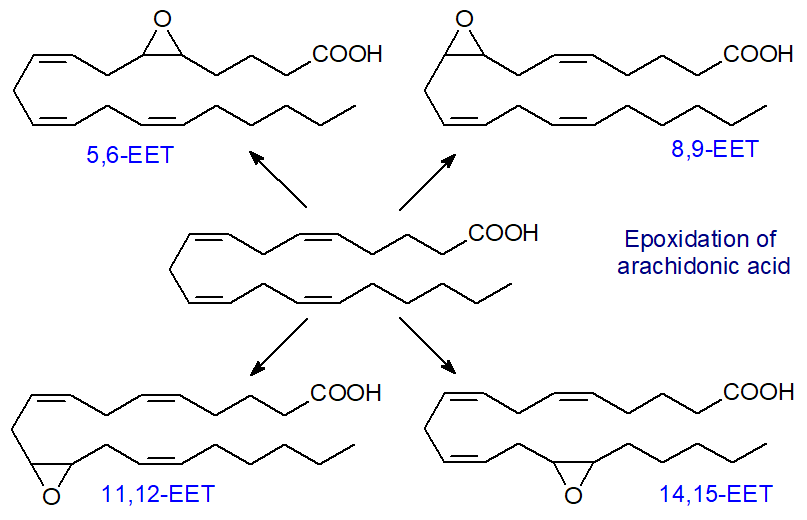
The proportions of the various isomers depend on tissue and species, although the 11,12- and 14,15‑EET generally tend to predominate. In the rat, 14,15‑EET amounts to about 40% of those produced in the heart, while 11,12-EET represents 60% of those produced in the kidney. To add to the complication, each of these regioisomers is a mixture of R,S- and S,R-enantiomers, and each iso-enzyme produces variable proportions, differing even among regioisomers. Eight isomers can be formed, therefore, each producing somewhat different responses in tissues. By the same means, adrenic acid (22:4(n-6)) can be converted to epoxy metabolites with 16,17‑epoxydocosatrienoic acid as the most abundant isomer in liver.
The epoxygenases require the fatty acid substrate to be in the unesterified form, but the products can be esterified later. Thus, significant amounts of epoxyeicosatrienes are found esterified to position sn-2 of phospholipids, including phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol, perhaps as a storage form that is available when a rapid response is required. Free epoxyeicosatrienes can then be released by phospholipase A2 after neuronal, hormonal or chemical stimulation, although it is possible that esterified epoxy-eicosanoids may have a function of their own within membranes. The presence of esterified EETs in plasma suggests that some exchange between tissues is likely, although most are believed to be produced close to the site of action. In many tissues, the esterified epoxy-eicosanoids are almost identical in composition to those in the free form, so the conclusion must be that they are entirely products of enzyme action. On the other hand, non-enzymic lipid epoxidation has been observed in erythrocytes in vitro, and some EETs with the epoxide group in both the cis- and trans-configurations may arise by this route.
EETs are rapidly metabolized in vivo to the corresponding dihydroxyeicosatrienoic acids (DHET) by epoxide hydrolases, of which at least five forms are known with different cellular locations and preferred substrates. The cytosolic or soluble (EPHX2 or sEH)) and membrane-bound (EPHX1) enzymes are of special importance, both in terms of oxylipin metabolism and for detoxification of xenobiotic epoxides. In humans, EPHX2 is widely expressed throughout the body and is a 62kDa enzyme composed of two domains separated by a short proline-rich linker in which the N‑terminal domain is a phosphatase towards lipid phosphates, while the C-terminal domain is the epoxide hydrolase. The reaction is illustrated below for the conversion of 14,15-EET to 14,15-DHET.
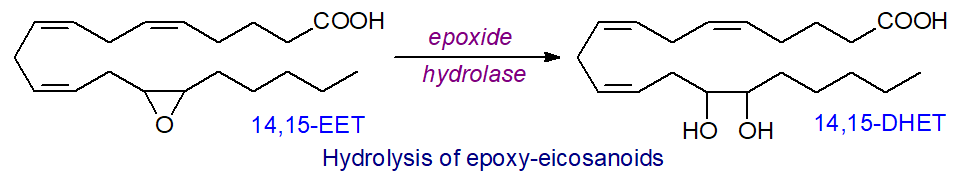
This enzyme metabolizes 8,9-, 11,12- and 14,15-EET efficiently, but 5,6-EET is a poor substrate. It displays some enantioselectivity, and this may be a factor in determining the stereochemistry of the circulating epoxides. 11,12- and 14,15-EET can undergo partial β‑oxidation to form C16 epoxy-fatty acids, or they can be elongated to C22 products, and 5,6- and 8,9-EET are substrates for cyclooxygenase. While DHETs were once believed to be merely inert products of EETs, they are now known to have appreciable effects of their own.
3. Oxo-Eicosatetraenoic Acids
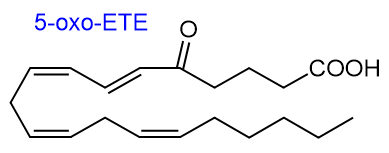 5-Oxo-6t,8c,11c,14c-eicosatetraenoic acid
(5-oxo-ETE) is a metabolite of 5S-hydroxy-6t,8c,11c,14c-eicosatetraenoic acid (5‑HETE),
produced by oxidation by an NADP+-dependent 5-hydroxy-eicosanoid dehydrogenase,
an enzyme found in the microsomal membranes of white blood cells (leukocytes), platelets, eosinophils and neutrophils (or by
transcellular biosynthesis from inflammatory cell-derived 5S-HETE).
The enzyme requires the presence of a 5S‑hydroxyl group and a
trans-6 double bond in the eicosanoid, and NADP+ is a cofactor.
Synthesis of the metabolite is stimulated during periods of oxidative stress, but some 5-oxo-ETE may be formed directly from
5‑hydroperoxyeicosatetraenoic acid, possibly by a non-enzymic route.
In neutrophils, a high proportion is rapidly incorporated into triacylglycerols.
5-Oxo-6t,8c,11c,14c-eicosatetraenoic acid
(5-oxo-ETE) is a metabolite of 5S-hydroxy-6t,8c,11c,14c-eicosatetraenoic acid (5‑HETE),
produced by oxidation by an NADP+-dependent 5-hydroxy-eicosanoid dehydrogenase,
an enzyme found in the microsomal membranes of white blood cells (leukocytes), platelets, eosinophils and neutrophils (or by
transcellular biosynthesis from inflammatory cell-derived 5S-HETE).
The enzyme requires the presence of a 5S‑hydroxyl group and a
trans-6 double bond in the eicosanoid, and NADP+ is a cofactor.
Synthesis of the metabolite is stimulated during periods of oxidative stress, but some 5-oxo-ETE may be formed directly from
5‑hydroperoxyeicosatetraenoic acid, possibly by a non-enzymic route.
In neutrophils, a high proportion is rapidly incorporated into triacylglycerols.
It appears that 5-hydroxyeicosanoid dehydrogenase can catalyse the reverse reaction, i.e., the reduction of 5-oxo-ETE, and this seems to be of special relevance in platelets. This reverse reaction changes the activity of 5-oxo-ETE, and alternatively, this can occur by reduction of the double bond in position 6, or by further oxidation either by lipoxygenases or by cytochrome P450 enzymes, the latter in positions 19 or 20.
All the HETE isomers can be converted to oxo-metabolites by hydroxy-eicosanoid dehydrogenases, and the 11-, 12- and 15‑isomers possess appreciable activity in tissues. 15S-HETE and 11R-HETE are substrates for 15‑hydroxyprostaglandin dehydrogenase, the enzyme catalysing the first step of prostaglandin catabolism, to yield 15-oxo-ETE and 11‑oxo‑ETE, respectively, which mediate anti-proliferative properties in endothelial cells. They can also form CoA esters, which can undergo up to four double bond reductions. 14‑Hydroxy-docosahexaenoic acid is a good substrate for the enzyme to yield the 14-oxo analogue. It is now recognized that α,β-unsaturated keto-eicosanoids generated in this way are electrophilic and have the potential to interact with nucleophilic centres in proteins and other molecules to modify their properties.
4. Mono-oxygenated Metabolites of EPA and DHA
Lipoxygenases and cytochrome P450 oxidases interact with the other essential polyunsaturated fatty acids of the omega-3 and omega-6 families, especially the former, to give comparable series of metabolites. Lipoxygenases have much the same positional specificities with eicosapentaenoic acid (EPA or 20:5(n-3)) as with arachidonic acid to produce hydroxy-eicosapentaenoic acids (HEPE), such as 5- and 12‑HEPE, but 18‑HEPE is produced by aspirin-acetylated COX-2 or by CYP2C8/CYP2J2. 5-Lipoxygenase generates 4- and 7‑hydroxy metabolites from docosahexaenoic acid (22:6(n‑3) or DHA), while 12-lipoxygenase generates 11- and 14-hydroxy metabolites, and 15‑lipoxygenase (15-LOX-2) introduces a 17‑hydroxyl group. These can react further to produce the protectins, resolvins and maresins or 'specialized pro-resolving mediators', which have special importance in the resolution of inflammation and have their own web page
The products of the lipoxygenases with arachidonate were soon documented, but it has taken longer to recognize the occurrence and properties of the metabolites of eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, e.g., the epoxides, produced by various cytochrome P450 oxidases. Indeed, it is now evident that these n-3 polyunsaturated fatty acids, rather than arachidonic acid, are the preferred substrates for some of the enzyme isoforms, namely the CYP1A, CYP2C, CYP2J and CYP2E subfamily members, which then exhibit very different regio- and stereo-specificities. Human CYP1A1 acts mainly as a subterminal hydroxylase with arachidonate to produce four different isomers, but with EPA it generates mainly 17R,18S-epoxy-eicosatetraenoate with almost absolute regio- and stereo-selectivity, and with DHA, it epoxidizes the n-3 double bond and produces 19,20‑epoxy-docosapentaenoate. Other isoforms of the cytochrome P450 enzymes produce epoxides by reaction with an n‑3 double bond in the same manner, some much more rapidly than with arachidonate as substrate, but CYP2C9 is an exception and oxidizes EPA to 14,15-epoxy-ETE mainly and DHA to 10,11‑epoxy‑DPE.
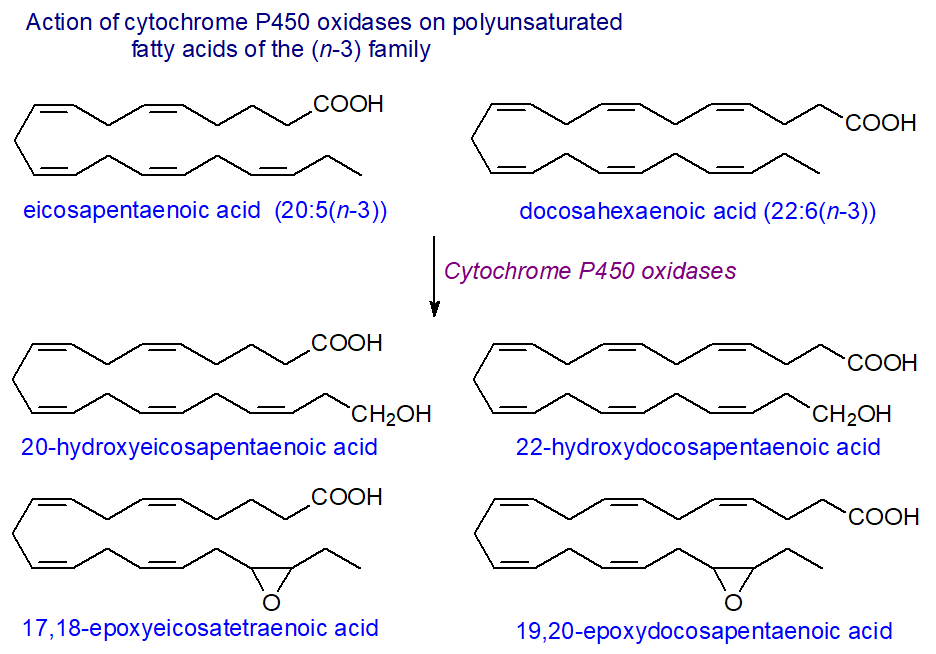
The CYP4A/CYP4F subfamilies generate 20-hydroxy-eicosatetraenoic acid from arachidonate in mammals, and they hydroxylate the terminal methyl group in EPA and DHA at the same rate. In addition, human endothelial cells with upregulated COX-2 and treated with aspirin convert EPA to 18R-hydroxyeicosapentaenoic acid with anti-inflammatory properties. 4‑Oxo‑DHA is present in plasma of rats fed DHA and is a potent anti-tumour agent against breast cancer, possibly because it can undergo the Michael reaction, although details of its fine structure and biosynthesis are awaited.
By competing with arachidonate, EPA and DHA may modify the action of the various HETE metabolites, but the oxygenated EPA and DHA compounds have biological properties of their own. For example, 17,18-epoxyeicosatetraenoic acid generated in the gut is an anti-allergic molecule, while significant amounts of DHA epoxides, mainly 7,8‑epoxydocosapentaenoic acid, are present in the central nervous system of rats, where they ameliorate inflammatory pain. It has been suggested that such EPA and DHA metabolites may be responsible for some of the benefits associated with dietary n‑3 fatty acid intake.
5. Octadecanoids
Linoleate hydroperoxides are produced in animal tissues by all the enzymes used in eicosanoid generation, including lipoxygenases, cyclooxygenases and cytochrome P450 enzymes, with production of octadecanoids or 'HODEs', and they can be catabolized by the same enzyme, i.e., 15-hydroxyprostaglandin dehydrogenase, to form keto derivatives. In the gut, bacteria produce 12(Z)-10- and 11(E)-10-hydroxyoctadecamonoenoic acids (HOME) by enzymatic oxidation of linoleic acid. While similar reactions occur with both α- and γ‑linolenic acids in vitro and their metabolites have been detected in plasma and some other tissues at low levels, relatively little appears to be known of their significance in vivo. Autoxidation of linoleate produces the same types of products but with more variable stereochemistry.
The action of lipoxygenases upon linoleic acid in plant tissues is discussed in the web page on plant oxylipins, but this fatty acid is acted upon by lipoxygenases in animal tissues in the same way to produce 9- and 13-hydroperoxy- and thence hydroxy-octadecadienoic acids of defined stereochemistry. 13S‑Hydroperoxy-9Z,11E-octadecadienoic acid (13S‑HPODE) is generated by the action of 15‑lipoxygenase (15‑LOX‑1) on linoleic acid, and this is reduced to the hydroxy compound, while oxo-, epoxy- and epoxy-keto-octadecenoic acids can be formed in further reactions, as illustrated. 12R-LOX reacts readily with linoleate (9,12-18:2) to produce 9R‑HPODE.
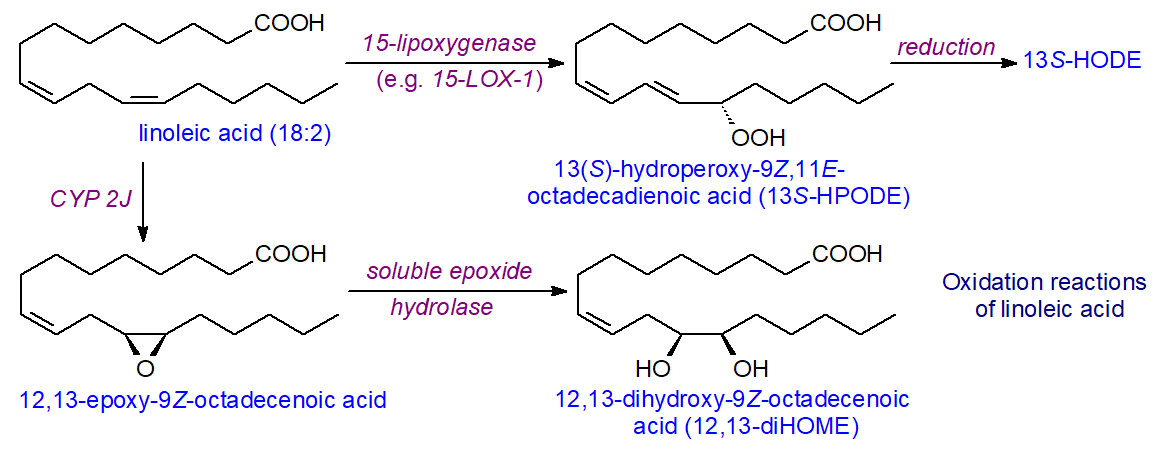
Enzymes of the cytochrome P450 family make a further contribution, and linoleic acid is a substrate for CYP epoxygenases, e.g., CYP2C9 in human liver, to yield the linoleic epoxides 9,10- and 12,13‑epoxyoctadecenoic acids, which are sometimes termed leukotoxins (although this name has been applied also to very different microbial metabolites). Epoxide hydrolase can then metabolize them to the 9,10- and 12,13‑diols, respectively. Epoxy-octadeca-monoenoic acids are produced by insects where they are believed to take part in the resolution of cellular and humoral immune reactions.
Linoleate metabolites were first found in patients with burns and inflammatory diseases, adult respiratory distress syndrome and chronic obstructive pulmonary disease (COPD), and the diols can cause mitochondrial-mediated cell death, although they can be detoxified by conversion to the glucuronides. On the other hand, 12,13-dihydroxy-9Z-octadecenoate (12,13-diHOME) synthesised in adipose tissue has beneficial properties (see below). As linoleic acid is a major unsaturated fatty acid in animal tissues, appreciable amounts of these hydroxy and hydroperoxy metabolites can accumulate and influence inflammatory diseases. Indeed, linoleate metabolites are by far the most abundant oxygenated fatty acids in both free and esterified form in human plasma and in the brain of rat pups.
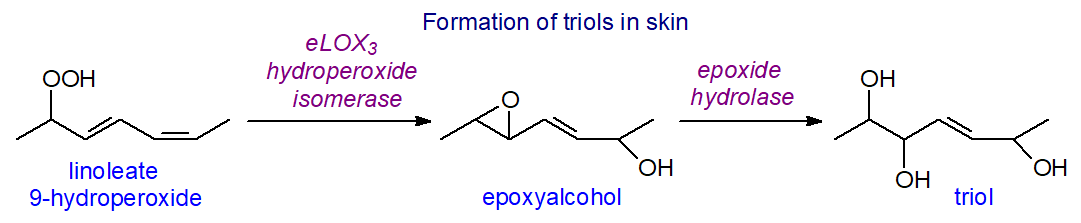
A further interesting observation is that one of the unique ceramides of skin, O‑linoleoyl-ω-hydroxyacyl-sphingosine, is a substrate for 12R‑LOX with 9R‑hydroperoxy-linoleoyl-ω-hydroxyceramide as the product. This in turn can be converted to hepoxilin-like compounds, i.e., with an epoxyl group, by an enzyme epidermal lipoxygenase 3 (eLOX-3), while trihydroxy compounds, e.g., octadec-9R,10S,13R-trihydroxy-11E-enoate (tri-HOMEs) may be formed subsequently by the action of an epoxide hydrolase, such as the human soluble enzyme. 9R,10S,13R-Trihydroxy-11E-octadecenoate is an important oxylipin formed in porcine and human epidermis, where it interacts with the ceramides to aid formation of the waterproof barrier. In the lung, tri-HOMEs are produced by a mechanism that is believed to start with formation of a 13S‑hydroperoxide by the action of 15-lipoxygenase and proceeds via an epoxide intermediate.
6. Esterified Oxylipins
Most eicosanoid-generating enzymes require free fatty acids as the substrate, and they are unable to oxidize intact phospholipids, although 15‑LOX in human monocytes is an exception (as is murine 12/15-LOX). However, free 5-, 12- and 15-HETEs can be esterified to phospholipids in tissues, often with some selectivity, as can hydroxydocosahexaenoic acids and hydroxyoctadecadienoic acids, and it has been established that all mammalian long-chain acyl-CoA synthetase isoforms have the capacity to activate HETE for further esterification through the action of membrane-bound O‑acyltransferases (thromboxanes may be an exception). The mechanisms for these esterification processes are discussed in our web page on oxidized phospholipids. It is evident that many of these reactions depend on particular cell types and lipids, and that cell compartmentalization is a significant factor, since eicosanoids of exogenous origin and those generated endogenously appear to be sensed differently. In contrast, non-enzymatic oxidation (autoxidation) of polyunsaturated fatty acids occurs when they are in esterified form by the initial steps described in our web page on isoprostanes.
15-HETE is selectively esterified to phosphatidylinositol in lung and kidney epithelial cells and in aortic endothelial cells, while 12-HETE occurs predominantly in phosphatidylcholine in microsomal membranes. In neutrophils, 5-HETE is incorporated mainly into phosphatidylethanolamine plasmalogens and phosphatidylcholine, while three 12‑hydroxyeicosatetraenoic acid phosphoinositides have been detected in thrombin-activated platelets. More than 90% of the EETs in most plasma and organ tissues are esterified to position sn‑2 of glycerophospholipids, and in aortic endothelial cells, 20‑HETE is present in esterified form in several phospholipid classes.
Many of these esterified lipoxygenase and oxidase products of phospholipids remain within the membranes where they are believed to serve as storage forms to be released on appropriate stimulation, possibly into other cellular compartments with different roles from their unesterified equivalents. On the other hand, such oxidized lipids have the potential to perturb membrane structures and carry out secondary oxygenations that could induce unwanted changes in cells, such as ferroptosis (see our web page on oxidized phospholipids), as discussed further below. Oxidation of low-density lipoprotein by this means has been implicated in the initiation of atherosclerosis, and there are suggestions that cholesteryl arachidonate in the lipoprotein LDL is a good substrate for 15‑LOX‑1 or oxidizing agents derived from it, and that the products are a causative factor in this disease (see our web page on cholesterol).
Phospholipids containing EET are substrates for the production of lipid mediators such as 2-epoxyeicosatrienoyl-sn-glycerols, analogous to the endocannabinoid 2-arachidonoylglycerol (and discussed further under this topic). Kidney and spleen, for example, synthesise sn-2-glycerol derivatives esterified with 11,12-EET or 14,15-EET, which are endocannabinoids and exert effects by their interaction with the CB1 and CB2 receptors, while phospholipids containing EET are probable substrates for synthesis of EET-ethanolamide in the liver and kidney. Endocannabinoids such as anandamide and synaptamide can be converted directly to various oxygenated derivatives, which can produce greater biological responses than their precursors.
7. Tissue Responses to HETE
Numerous hydroxyeicosatetraenoic acids and related compounds have now been discovered and most of these have some form of biological activity, in vitro at least, and primarily in signalling. They modulate ion transport, vascular tone, renal and pulmonary functions, and growth and inflammatory responses through both receptor and non-receptor mechanisms. Their release is stimulated by the action of growth factors and cytokines, and they attain physiological concentrations in tissues that are much higher than those of prostanoids. This is a field that is still developing rapidly, and it is evident that the picture is complex and very far from complete. A given eicosanoid of this kind can have differing functions in different cell types, and as this may be opposed or modified by another eicosanoid, the balance between them in a cell may be critical. As animal models can have very different isoforms of enzymes, it is often difficult to translate experiments with other species to human conditions. It is not possible to give a comprehensive picture of this complexity here, as this would require a substantial tome, so only a few of best known and studied are described briefly below.
HETEs: 5S-Hydroxy-6t,8c,11c,14c-eicosatetraenoic acid (5S-HETE) is the precursor of the leukotrienes and lipoxins, but it has some functions in its own right, although these can be difficult to disentangle from those of its metabolites. Like its metabolite 5-oxo-HETE, 5S‑HETE activates neutrophils and monocytes, and it is known to stimulate proliferation of cancer cells in a similar manner to certain leukotrienes with increased amounts formed in brain tumours; 5-LOX inhibitors are preventive.
5-Oxo-6t,8c,11c,14c-eicosatetraenoic acid is a chemo-attractant for eosinophils and neutrophils, and it also takes part in actin polymerization, calcium mobilization, integrin expression and degranulation. Its signalling functions are mediated via a Gi/o-coupled receptor ('OXE'), leading to increased intracellular calcium concentrations and inhibition of cAMP production. By increasing the production of dermal fibroblasts, it promotes wound healing, but this is inhibited by the action of ceramide 1-phosphate on its receptor (OXER1 in mice). In contrast, it stimulates the proliferation of prostate tumour cells, and it is believed to be a mediator in asthma and other allergic diseases. Efforts are underway to find inhibitors of the OXE receptor of potential clinical value.
 Arachidonate
8S-lipoxygenase and its product 8S-hydroxy-5c,9t,11c14c-eicosatetraenoic
acid (8S-HETE) has only been found in the skin of mice.
It is a potent activator of the peroxisome proliferator-activated receptor PPARα, it is an anti-tumorigenic agent towards skin cancer,
and it promotes wound healing in the cornea.
In contrast, a human orthologue of this enzyme (15‑LOX‑2) is found in skin, sebaceous glands and prostate tissue but produces
15S-HETE.
Arachidonate
8S-lipoxygenase and its product 8S-hydroxy-5c,9t,11c14c-eicosatetraenoic
acid (8S-HETE) has only been found in the skin of mice.
It is a potent activator of the peroxisome proliferator-activated receptor PPARα, it is an anti-tumorigenic agent towards skin cancer,
and it promotes wound healing in the cornea.
In contrast, a human orthologue of this enzyme (15‑LOX‑2) is found in skin, sebaceous glands and prostate tissue but produces
15S-HETE.
12S-Hydroxy-5c,8c,10t,14c-eicosatetraenoic acid (12S-HETE) is the precursor of the hepoxilins but has functions of its own. In nervous tissue, it modulates membrane properties and stimulates melatonin synthesis, while together with 15S‑HETE, it serves as a secondary messenger in synaptic transmission and is involved in learning and memory processes; increased levels are found in Alzheimer's disease. 12S‑HETE can either stimulate or inhibit aggregation in platelets, depending on species and circumstances, and it can cause constriction of blood vessels but inhibition of thromboxane (TxA2)-induced platelet aggregation while stimulating lipoxin synthesis. The serum level of 12-HETE has been shown to be elevated in individuals with coronary heart disease, and in leukocytes, it promotes chemotaxis and induces the synthesis of heat-shock protein.
Particular attention has been devoted to how 12S‑HETE suppresses cancer cell apoptosis, and promotes cancer cell invasion, motility, and tumour angiogenesis. It inhibits the adhesion of cancer cells to endothelial cells, and this has been linked to metastasis in cancer of the prostate and is mediated via cell surface signalling and activation of protein kinase C; it promotes the proliferation of ovarian and other cancer cells by various mechanisms. Extracellular vesicles derived from platelets promote tumour metastasis, and the explanation appears to be that they deliver 12‑lipoxygenase to cancer cells where free and phospholipid-esterified 12S‑HETEs are generated.
The enantiomeric compound 12R-HETE is believed to be a factor in the pathophysiology of psoriasis and related skin diseases, although it is crucial for the development of normal skin. 12R-HETE produced by cytochrome P450 enzymes may have a function in the eye. 12S‑Hydroxy-8,10,14-eicosatrienoic acid (12S‑HETrE), derived from dihomo-γ-linolenic acid (20:3(n‑6)), has been shown to provide protection against thrombotic-mediated events in vivo and has a potential therapeutic role in providing cardioprotection by interacting with the prostacyclin receptor (IP).
The hydroperoxide precursors of the various HETE isomers tend to be less studied, but 12S-hydroperoxy-ETE is reported to be a key player in oxidative stress in platelets and is known to stimulate the metabolism or arachidonate and other polyunsaturated fatty acids by activating phospholipase A2 (cPLA2) and cyclooxygenase (COX-1), the first enzymes required for prostanoid production.
15S-Hydroxy-5c,8c,11c,13t-eicosatetraenoic acid (15S-HETE) is a precursor of the lipoxins and is produced by two lipoxygenases in human tissues, one of which (15-LOX-1) is related structurally to the 12-lipoxygenase of leukocytes and is unusual in that it produces some 12-HETE in addition to the 15‑isomer. The second form of the enzyme was first found in the epidermis, although it is now known to exist in other tissues. 15S-HETE has been implicated in cell differentiation, inflammation, asthma and carcinogenesis. In atherogenesis, there is an accumulation of 15-HETE in human carotid plaques, and this is believed to play a part in the induction of atherothrombotic events by increasing platelet aggregation and thrombin generation. 15‑HETE appears to contribute to the development of Hodgkin lymphoma, colorectal, lung and many other cancers, but that produced by 15‑LOX‑2 activates PPARγ, a nuclear transcription factor involved in epithelial differentiation, which may explain an anti-proliferative action on prostate cancer cells. 15-LOX-1 is a feature of the processes of apoptosis, autophagy and ferroptosis, and reduced levels of this enzyme in some cancers lead to decreased activity of PPARγ, resulting in a halt to apoptosis and enhanced cell proliferation.
Of the terminal and near-terminal HETE isomers (cytochrome P450 metabolites), 20-HETE has been considered to be pro-inflammatory and can be detrimental by increasing hypertension, promoting systemic vasoconstriction and tumour growth, while it may be a factor in rheumatoid arthritis. It regulates vascular smooth muscle and endothelial cells by influencing their proliferation, migration, survival and tube formation, acting via the G protein receptor GPR75. In contrast, 20-HETE has the potential to prevent septic shock and multi-organ failure induced by bacterial lipopolysaccharides. It exerts a beneficial effect in terms of insulin secretion, and in the kidney, it is anti-hypertensive by blocking re-absorption of sodium by inhibiting the Na+‑K+‑ATPase, although it has been implicated in the pathogenesis of other kidney diseases. Production is increased after the onset of both ischemic and hemorrhagic strokes. Although 20‑HETE may promote tumour growth, 8- and 11‑HETE are anti-tumour agents. EPA and DHA are potent inhibitors of the biosynthesis of 20‑HETE, suggesting that this may be a partial explanation for the physiological role of omega-3 fatty acids, although other HETE isomers appear to act in opposition to it, and 18- and 19‑HETE induce vasodilatation by inhibiting 20‑HETE. Together with 16- and 17‑HETE, they induce re-uptake of sodium in the kidney, while 16‑HETE inhibits neutrophil adhesion so may be relevant to inflammation. On the other hand, high 19-HETE concentrations have been correlated with cardiovascular events.
The 3-hydroxy-eicosanoids produced by pathogenic fungi may take part in the inflammatory processes associated with infections by such organisms, as they are strong pro-inflammatory lipid mediators. As they are produced during the reproductive phase of yeast and fungal growth, the organisms presumably require them for their own internal purposes.
Metabolites of EPA and DHA: The EPA lipoxygenase metabolite 5-hydroxy-eicosapentaenoic acid (5-HEPE) enhances the induction of regulatory T cells (Tregs) that modulate the immune system and prevent autoimmune disease, and it can increase insulin secretion from pancreatic beta cells in mice. Both 5- and 12-HEPE are induced in brown fat when exposed to cold and have been termed 'batokines' or 'lipokines' that improve glucose metabolism by promoting glucose uptake into adipocytes and skeletal muscle through triggering an insulin-like intracellular signalling pathway. Apart from being a precursor of E-series resolvins, 18‑HEPE per se has cardioprotective properties and inhibits metastasis in a cancer model. Various HEPE isomers have been detected in psoriatic arthritis, where they are believed to be anti-inflammatory.
It is noteworthy that while 17-hydroxy-DHA derived from the action of 15-LOX is often considered simply as a precursor of the specialized pro-resolving mediators, there is evidence that it rather than the latter alleviates the sensitivity to heat pain and osteoarthritis pain in humans. In brain, 7S-HDHA is a high-affinity PPARα ligand that stimulates the growth of neurons and regulates the expression of genes associated with their morphology. In obese mice, 17‑HDHA attenuated inflammation in adipose tissue and improved insulin sensitivity and glucose tolerance.
 Epoxyeicosatrienoic acids (EETs): The various EETs are crucial autocrine and paracrine effectors
in the cardiovascular and renal systems that are believed to be anti-inflammatory and largely beneficial.
As the regioisomers and enantiomeric forms have many similar metabolic and functional properties, epoxyeicosatrienoic acids have often been
treated as a single class of compounds, although as knowledge has expanded this view is no longer justifiable.
11,12-EET in particular induces distinctive responses in tissues.
Epoxyeicosatrienoic acids (EETs): The various EETs are crucial autocrine and paracrine effectors
in the cardiovascular and renal systems that are believed to be anti-inflammatory and largely beneficial.
As the regioisomers and enantiomeric forms have many similar metabolic and functional properties, epoxyeicosatrienoic acids have often been
treated as a single class of compounds, although as knowledge has expanded this view is no longer justifiable.
11,12-EET in particular induces distinctive responses in tissues.
Because of the anti-hypertensive, anti-fibrotic and anti-thrombotic properties of EETs, their presence in red blood cells has implications for the control of circulation and the physical properties of the circulating blood. Both cis- and trans-EETs are synthesised and stored in erythrocytes, and they are produced and released in response to a low oxygen concentration as during exercise. 11,12‑EET enhances the process by which immature precursor cells develop into mature blood cells (hematopoiesis) and in their further development (engraftment) in mice and zebrafish in vitro. In the kidney, EETs modulate ion transport and gene expression to produce vasodilation, and in other tissues, they exert beneficial effects on insulin resistance and obesity-associated diseases, they alleviate inflammatory pain, neuroinflammation and neuroinflammatory diseases, and they improve lung function and wound healing. By direct and indirect anti-inflammatory actions in the myocardium, they alleviate cardiomyopathy and cardiac remodelling. There is a suggestion that signalling by 11,12-EET may be a factor in the regulation of the response to DNA damage, and it is reported to beneficial towards pulmonary fibrosis. Through interactions with select binding pockets, fatty acid binding proteins (FABP3 and FABP5) modulate signalling of EETs in synapses in the brain. As significant amounts of EETs are incorporated into phospholipids from which they are rapidly released in the presence of Ca2+ ionophores, it has been suggested that they may take part in those signal transduction processes mediated by phospholipases.
For some purposes, epoxy-eicosanoids require cell-surface receptors, and GPR132 has been identified as a potentially low-affinity EET receptor with physiological relevance in hematopoiesis, but they can also make use of intracellular mechanisms, i.e., direct interaction with ion channels, signalling proteins or transcription factors. In the central nervous system, epoxyeicosanoids regulate the release of neurohormones and neuropeptides, and by reducing the long-term damage associated with central neurologic insults, they may be beneficial towards neurologic diseases including Parkinson's disease, Alzheimer's disease and dementia. Their concentrations are controlled by soluble epoxide hydrolases, and it is hoped that inhibitors of these will be developed with therapeutic potential against several debilitating inflammatory diseases. In non-failing human hearts, one isoform of phospholipase A (cPLA2ζ) channels arachidonic acid into protective EETs, whereas in failing hearts, opening of the mitochondrial permeability transition pore increases the activity of a second isoform of phospholipase A (cPLA2γ) that channels arachidonic acid into toxic HETEs.
 17,18-Epoxyeicosatetraenoic acid is the main epoxide regio-isomer synthesised
from eicosapentaenoic acid and has anti-allergy and anti-inflammatory properties by activating the receptor GPR40;
it may have therapeutic properties in the skin and intestines.
In sensory neurons, it acts through the prostacyclin receptor (IP) to sensitize the transient receptor potential vanilloid 1 (TRPV1).
It is a vasodilator and may be responsible for some of the beneficial effects of dietary omega-3 fatty acids.
While 19,20-epoxy-docosapentaenoic acid derived from DHA has been shown to be beneficial in tissues,
much less is known of the other oxygenated metabolites of EPA and DHA, although they appear to act in opposition to HETE
isomers and may be important in the cardiovascular system and as anti-cancer agents.
17,18-Epoxyeicosatetraenoic acid is the main epoxide regio-isomer synthesised
from eicosapentaenoic acid and has anti-allergy and anti-inflammatory properties by activating the receptor GPR40;
it may have therapeutic properties in the skin and intestines.
In sensory neurons, it acts through the prostacyclin receptor (IP) to sensitize the transient receptor potential vanilloid 1 (TRPV1).
It is a vasodilator and may be responsible for some of the beneficial effects of dietary omega-3 fatty acids.
While 19,20-epoxy-docosapentaenoic acid derived from DHA has been shown to be beneficial in tissues,
much less is known of the other oxygenated metabolites of EPA and DHA, although they appear to act in opposition to HETE
isomers and may be important in the cardiovascular system and as anti-cancer agents.
Until recently, EETs were believed to be relatively benign molecules, but it has now been demonstrated in mice that they are stimulants for the release of primary cancer tumours from dormancy, for promoting their growth and for triggering metastasis, i.e., the spread of cancer to other organs; their dihydroxy metabolites have even stronger effects upon cancer progression. The dihydroxyeicosatrienoic acids (DHET) produced by epoxide hydrolases are pro-inflammatory in general, and they have been associated with colonic inflammation in obese mice and with osteoarthritis in the knee in humans. Acting via ferroptosis, dihydroxy-metabolites of dihomo-γ-linolenic acid (20:3(n-6)) are reported to cause neurodegeneration, while 19,20‑dihydroxydocosapentaenoic acid has a role in the development of diabetic retinopathy. As dihydroxy metabolites may be factors in psychiatric and neurological disorders, the soluble epoxide hydrolase (EPHX1) is a therapeutic target for such conditions as well as to assist in cardiac recovery after ischemia. Inhibitors of the enzyme are undergoing clinical trials for several inflammatory conditions, including cancer.
Octadecanoids: The oxidized linoleate metabolites 13S-HODE and 9S-HODE are believed to be atherogenic through the induction of pro-inflammatory cytokines and formation of foam cells from macrophages by activation of PPARs, e.g., PPARγ, and other receptors. 9S‑HODE but not 13S-HODE is a high-affinity ligand of GPR132 and is proinflammatory, and the former is a marker for oxidative stress and contributes to the process of pain perception. In a Drosophila model, it regulates the FOXO family of transcription factors. HODE are found at increased levels in psoriatic skin, and they contribute to hepatic injury, including non-alcoholic steatohepatitis, possibly by non-enzymatic formation of potentially toxic adducts with proteins. As the actions of octadecanoids on the regulation of inflammation are of relevance to the metabolic processes associated with atherogenesis and cancer, they are attracting special interest.
In contrast, there is evidence that a 15-LOX metabolite 13S-HPODE induces apoptosis in colon cancer cells. 13S-HODE is the brains of rat pups is reported to increase axonal outgrowth cortical neurons in male rat pups significantly, but not in female pups where linoleic acid per se acted in this way. In general, in brain, HODE regulate pain thresholds, inflammation, neurotransmission and the response to ischemic brain injury.
Epoxy-octadecenoic acids (epOMEs) are metabolites of linoleate from the cytochrome P450 monooxygenase pathway that are cancer-promoting in the colon via mechanisms that involve the gut microbiota. At high levels, the dihydroxy-metabolites (DiHOMEs) of these are vascular permeability and cytotoxic agents associated with multiple organ failure, adult respiratory distress syndrome, and sepsis in burn patients, although they are not implicated in colon cancer. In severe burn injury, DiHOMEs drive immune cell dysfunction through hyperinflammatory neutrophilic and impaired monocytic actions, so inhibition of soluble epoxide hydrolase may be a promising therapeutic strategy. 12,13‑Dihydroxy-9Z-octadecenoic acid (12,13‑diHOME) causes increased sensitivity to inflammatory pain and has been associated with the development of paediatric asthma, while both 9,10- and 12,13-diHOME are reported to be biomarkers for small versus large vessel stroke, together with blood brain barrier and neurovascular-glial disruption and temporal lobe atrophy. 9,10-DiHOME produced by commensal bacteria in the intestines facilitates regulatory T cell differentiation and may be a biomarker for colitis.
On the other hand, synthesis of 12,13‑diHOME is induced by cold with the effect of stimulating brown adipose tissue by promoting the uptake of fatty acids. As plasma levels are increased by exercise and the source is believed to be brown adipose tissue, 12,13-diHOME has been termed an exercise-stimulated lipokine (or batokine), which produces an increase in fatty acid oxidation and uptake in skeletal muscle that results in improved whole-body metabolic homeostasis and may be cardioprotective. It has been detected in human milk where it is believed to influence infant adiposity. In contrast, 12,13-diHOME (and noradrenaline) have been correlated with the occurrence of acute myocardial infarction and cognitive decline in patients with type 2 diabetes mellitus.
Hepoxilin-like triHOMEs are important in skin, where they are vital for epidermal barrier formation (and some argue for mammalian survival), but their levels are dysregulated in asthma and chronic obstructive pulmonary disease (COPD). In psoriasis, 9,10‑epoxy-13-hydroxy-octadecenoate and 9,10,13-trihydroxy-octadecenoate contribute to itch and pain.
Oxidized phospholipids: Although studies are at a relatively early stage, it is apparent that esterified HETEs have their own functions in relation to apoptosis, immune regulation, signalling and blood coagulation. Oxidation of cardiolipin by an enzyme of the cytochrome c family is an significant event in triggering mitochondrial apoptosis, while phosphatidylserine containing oxidized fatty acids is externalized to the surface of cells and is a signal for engulfment and digestion of apoptotic cells. Phosphatidylethanolamines containing arachidonic and adrenic acids that are oxidized by 15-LOX are believed to be pro-ferroptotic signals, but oxidized phosphatidylcholine is reported to be anti-inflammatory and protective in the lung. 12S‑HETE-lysophospholipids react selectively with certain human monocytes to generate tumour necrosis factor α (TNFα) and thence initiate a key signalling pathway; they may serve as biomarkers for age-related diseases and could potentially be used as targets for therapeutic intervention. Indeed, dysregulation of the metabolism of any of these molecules may have implications for human health. Further effects are discussed in our web page on oxidized phospholipids, including those produced non-enzymatically.
8. Fungal and Bacterial Enzymes
Certain fungi and yeasts produce 3R- and/or 3S-HETE and 3,18-di-HETE, when supplied with exogenous arachidonic acid from their animal hosts during infection. The biochemical mechanism is unclear, and there are reports that implicate lipoxygenases, cyclooxygenases or mitochondrial β-oxidation. With some pathogenic fungi, the 3-hydroxyeicosanoids produced in infected cells can be acted upon by the host COX-2 enzyme to form a family of 3-hydroxy-prostaglandins, which are at least as active biologically as the normal compounds. Aspergillus fumigatus has two lipoxygenase homologues of human ALOX15 and ALOX5, termed LoxA and LoxB, of which the latter is secreted extracellularly and produces 13‑hydroxyoctadecadienoic acid (13‑HODE) from linoleate.
Some fungi produce peroxygenases that introduce oxygen atoms into non-activated carbon-hydrogen bonds of aliphatic and aromatic compounds. As they only require H2O2 for catalysis and not cofactors and complex regeneration systems, they have great biotechnological potential.
Prokaryotic LOX-encoding genes are best known from Pseudomonas aeruginosa (Gram-negative bacteria) with the capacity to produce 15S‑hydroxyeicosatetraenoic acid (15S‑HETE), but these are distant evolutionarily from human LOX. This species has a cytochrome P450 enzyme, CYP168A1, which is a subterminal fatty acid hydroxylase that can produce 19-HETE from arachidonic acid. Many other bacterial species are now known to produce lipoxygenases, and some of these can utilize oleic, linoleic or polyunsaturated fatty acids as substrates with high positional and stereochemical specificity, while others, including Rhodococcus sp. and Bacillus megaterium, produce monooxygenases of the P450 family.
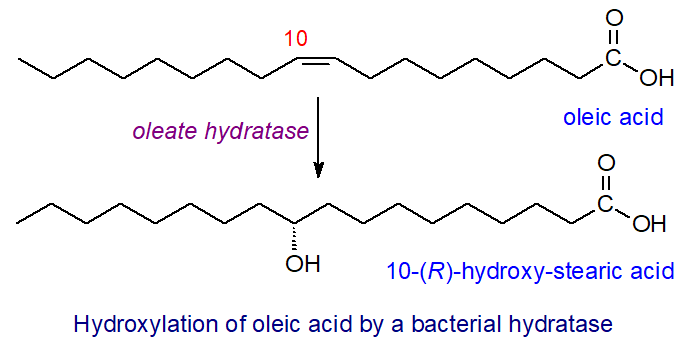
Fatty acid hydratases are present in bacteria but not in Eukaryotes, and these oxidize fatty acids by adding the elements of water to double bonds, with flavin adenine dinucleotide (FAD) as a cofactor, presumably incidental to the detoxification of environmental toxins. For example, oleate hydratases, such as that from Staphylococcus aureus, catalyse the hydroxylation of oleic acid to 10R-hydroxy-stearic acid, a reaction that may prove useful in industry.
9. Analysis
The remarkable array of eicosanoids and related oxygenated metabolites in animal tissues provides a daunting task for analysts, partly because of their sensitivity to any manipulation at low natural concentrations and partly because of a lack of suitable standards. Selective extraction, concentration and derivatization steps are required, followed by gas chromatography or high-performance liquid chromatography linked to mass spectrometry. Of the many published procedures, the most comprehensive appears to be that of Wang, Y. et al. (2014) cited below, who describe the analysis of 184 oxylipins in a single chromatographic run in only five minutes, with the use of deuterated internal standards and tandem mass spectrometry to ensure accurate quantification (I have no personal experience in this area). Equally authoritative for the analysis of octadecanoids is Quaranta, A. et al. (2022).
Recommended Reading
- Aranda, C., Carro, J., Gonzalez-Benjumea, A., Babot, E.D., Olmedo, A., Linde, D., Martinez, A.T. and Gutierrez, A. Advances in enzymatic oxyfunctionalization of aliphatic compounds. Biotechn. Adv., 51, 107703 (2021); DOI.
- Benatzy, Y., Palmer, M.A. and Brüne, B. Arachidonate 15-lipoxygenase type B: Regulation, function, and its role in pathophysiology. Front. Pharm., 13, 1042420 (2022); DOI.
- Biringer, R.G. The enzymology of human eicosanoid pathways: the lipoxygenase branches. Mol. Biol. Rep., 47, 7189-7207 (2020); DOI.
- Biringer, R.G. A review of non-prostanoid, eicosanoid receptors: expression, characterization, regulation, and mechanism of action. J. Cell Commun. Signal., 16, 5-46 (2022); DOI.
- Chrisnasari, R., Hennebelle, M., Vincken, J.P., van Berkel, W.J.H. and Ewing, T.A. Bacterial lipoxygenases: Biochemical characteristics, molecular structure and potential applications. Biotech. Adv., 61, 108046 (2022); DOI.
- Eccles, J.A. and Baldwin, W.S. Detoxification cytochrome P450s (CYPs) in families 1-3 produce functional oxylipins from polyunsaturated fatty acids. Cells, 12, 82 (2023); DOI.
- Hajeyah, A.A., Griffiths, W.J., Wang, Y., Finch, A.J. and O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol., 11, 591819 (2020); DOI.
- Lagarde, M. and Nicolaou, A. (Editors) Oxygenated metabolism of PUFA: analysis and biological relevance. Biochim. Biophys. Acta, Lipids (Volume 1851, Issue 4, Pages 307-518) (2015) - special issue.
- Lin, L., Dai, F., Wei, J.J. and Chen, Z. Biological roles of 5-oxo-6,8,11,14-eicosatetraenoic acid and the OXE receptor in allergic diseases: Collegium Internationale Allergologicum Update (2024). Int. Arch. Allergy Immunol., 185, 301-310 (2024); DOI.
- McReynolds, C., Hammock, B. and Morisseau, C. Regulatory lipid vicinal diols counteract the biological activity of epoxy fatty acids and can act as biomarkers and mechanisms for disease progression. Pharmacol. Therapeut., 248, 108454 (2023); DOI.
- Mendoza, S.R., Zamith-Miranda, D., Takács, T., Gacser, A., Nosanchuk, J.D. and Guimarães, A.J. Complex and controversial roles of eicosanoids in fungal pathogenesis. J. Fungi, 7, 254 (2021); DOI.
- Meng, Y.-W. and Liu, J.-Y. Pathological and pharmacological functions of the metabolites of polyunsaturated fatty acids mediated by cyclooxygenases, lipoxygenases, and cytochrome P450s in cancers. Pharmacol. Therapeut., 2561, 108612 (2024); DOI.
- Ni, K-D. and Liu, J.Y. The functions of cytochrome p450 ω-hydroxylases and the associated eicosanoids in inflammation-related diseases. Front. Pharm., 12, 716801 (2021); DOI.
- Oliw, E.H. Thirty years with three-dimensional structures of lipoxygenases. Arch. Biochem. Biophys., 752, 109874 (2024); DOI.
- Quaranta, A., Revol-Cavalier, J. and Wheelock, C.E. The octadecanoids: an emerging class of lipid mediators. Biochem. Soc. Trans., 50, 1569-1582 (2022); DOI.
- Quaranta, A. and 12 others Development of a chiral supercritical fluid chromatography-tandem mass spectrometry and reversed-phase liquid chromatography-tandem mass spectrometry platform for the quantitative metabolic profiling of octadecanoid oxylipins. Anal. Chem., 94, 14618-14626 (2022); DOI.
- Sarparast, M., Dattmore, D., Alan, J. and Lee, K.S.S. Cytochrome P450 metabolism of polyunsaturated fatty acids and neurodegeneration. Nutrients, 12, 3523 (2020); DOI.
- Shi, Z.Q., He, Z.W. and Wang, D.W. CYP450 epoxygenase metabolites, epoxyeicosatrienoic acids, as novel anti-inflammatory mediators. Molecules, 27, 3873 (2022); DOI.
- Singh, N.K. and Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res., 73, 28-45 (2019); DOI.
- Wang, B., Wu, L.J., Chen, J., Dong, L.L., Chen, C., Wen, Z., Hu, J., Fleming, I. and Wang, D.W. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transd. Targ. Ther., 6, 94 (2021); DOI.
- Zhou, M.Z., Li, J.D., Xu, J.Y., Zheng, L.F. and Xu, S.T. Exploring human CYP4 enzymes: Physiological roles, function in diseases and focus on inhibitors. Drug Discovery Today, 28, 103560 (2023); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: May 15th, 2024 | ||
